![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
117 Cards in this Set
- Front
- Back
|
Name and describe hormones from hypothalamus. (6)
|
1. Thyrotropin-releasing hormone (TRH)
Stimulus for TSH synthesis Only used for diagnostic purposes 2. Gonadotropin-releasing hormone (GnRH) Stimulus for FSH/LH synthesis and secretion For therapeutic and diagnostic use Can be used to induce ovulation 3. Corticotropin-releasing hormone (CRH) Regulates ACTH from ant. Pit. 4. Growth hormone-releasing hormone (GHRH) – aka sermorelin Stimulus for GH Diagnostic uses 5. Somatostatin (GH-RIH) Inhibitory control of GH release 6. Prolactin-inhibiting factor – probably dopamine Only control on prolactin release |
|
|
Name hormones from anterior pituitary gland (6). How are they grouped? (3 groups)
|
No sugar group
1. GH 2. Prolactin Glycoproteins 1. TSH or thyrotropin 2. LH 3. FSH group 3 1. ACTH (corticotropin) all are peptide hormones |
|
|
Name and describe hormones from posterior pituitary gland (2).
|
ADH (vasopressin) - bind to V2 receptors and promote water retention
Oxytocin - promote milk ejection and uterine contraction during labor |
|
|
Which hormone is made from AA tyrosine?
|
Thyroxine (T4)
triiodothyroinine (T3) |
|
|
Where is the receptor for T3 located in the cell?
Is the onset of action fast or slow? |
in the cytosol.
T3 binds to intracellular enzyme and works as a Tx factor --> slow onset of action |
|
|
What is the general difference b/t long-loop and short-loop negative feedback?
|
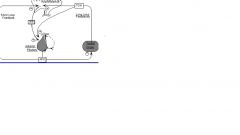
Long-loop negative feedback
i) High[ hormones] from the target organ --> inhibit hypothalamus and anterior pit Low hormones --> disinhibit hypothalamus and anterior pit Short-loop negative feedback Products of ant pit. Inhibits itself Another example using glucose (1) High glucose --> release insulin (2) Low glucose --> release glucagon |
|
|
Which feedback loop is most important in the hypo-ant. pit-thyroid gland axis?
|
Long-loop negative feedback from thyroid hormone to anterior pituitary gland.
|
|
|
What are actions of iodide on the thyroid gland at normal level? super high level?
|
normal - stimulatory effect
super high - shut down thyroid hormone synthesis |
|
|
what are 3 main forms of goiters in hypothyrodism? Etiology of each?
|
1. Endemic goiters caused by lack of sufficient iodide intake, leading to depressed T4 and T3 levels
(1) increased TSH leads to large increase in gland size 2. idiopathic nontoxic goiters iodide intake sufficient but hormone secretion diminished (1) usually caused by thyroiditis leading to a slight hypothyroid condition (2) in most cases thyroiditis based on autoimmune mechanisms 3. Colloid goiters with enzyme abnormalities |
|
|
What is myxedema? How is this related to the activities of thyroid hormone?
|
myxedema is an accumulation of protein in the interstitial spaces
Myxedema coma signifies the end stage of untreated hypothyroidism (acute medical emergency) |
|
|
What is cretinism? cause?
|
Insufficient growth due to hypothyroidism during fetal life or childhood.
(can be restored at any time with thyroid hormones but mental retardation results w/o prompt replacement) |
|
|
Name 2 synthetic thyroid hormones? What is the main difference b/t the two?
3 drug interactions with thyroid hormones? |
Levothyroxine - slower onset of action
Liothyronine - has a faster onset of activity; used for acute disorders 1. coumarin anticoagulants i) thyroid hormone --> effects of coumarin ii) when on both --> prescribe less coumarin 2. cholestyramine (BAS) i) Cholestyramine and bind T3 and T4 in GI and slow absorption ii) Take the drugs 4-5hrs apart 3. enzyme inducing drugs i) e.g. insulin |
|
|
Liothyronine
what is it? Indication? Contraindication? Advantage/disadvantage? |
Synthetic T3
Indicated for acute hypothyroidic disorders such as myxedema contraindicated for CV disease patients Advantage - fast onset of action disadvantage - short half-life |
|
|
Levothyroxine
What is it? Indication? Contraindication? |
Synthetic T4
Indication - preferred drug for hypothyroidism (long-term) contraindication - CV disease |
|
|
What drug is often prescribed with thyroid hormone therapy in CV patients?
|
Propanolol - beta blocker
|
|
|
List 3 forms of hyperthyroidism
|
1. Hyperplastic thyroid
2. Grave's disease 3. Thyroid adenoma |
|
|
List 3 treatment choices for hyperthyroidism.
|
1. Antithyroid drugs (Propylthiouracil/PTU, methimazole)
2. Radiation 3. Surgery |
|
|
Name 2 Antithyoid drugs.
Difference b/t 2? MOA? Indications? |
Propylthiouracil and methimazole
PTU - more widely used Methimazole - longer T1/2 and widely used MOA - Inhibits Iodide incoporation into thyroglobulin; decrease peripheral T4 to T3 conversion Indications: preferred for Grave's, pregnant, children Prior to surgery or radiation to decrease metabolic rate Pref |
|
|
high dose Iodide for hyperthyroidism
MOA Indications disadvanges |
MOA - inhibits synthesis and release of thyroid hormone
Indications - prior to surgery in conjunction with antithyroid drugs disadvantage - fast desensitization (few months) |
|
|
What is thyrotoxicosis?
symptoms? Recommended treatment? rationale? |
Thyrotoxicosis - thyroid storm; may result in hyperthyroid patients
Symptoms - hyperthermia, tachycardia, heart failure and syncope Antithyroid drug first b/c it prevents T4 to T3 conversion Iodide, propranolol, glucocorticoids later. |
|
|
What is the main control of glucocorticoid release?
What can override this control? |
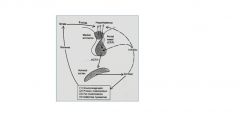
ACTH is the primary control for glucocorticoid released; in sync with circadian rhythm
Acute stress can override ACTH |
|
|
What is the main control of mineralocorticoid release?
Minor ones? (2) |

Main control - low plasma [K+] --> release of mineralocorticoid
minor controls 1. renin-angiotensin system 2. ACTH has a permissive effect on the release |
|
|
List 3 actions of mineralocorticoids.
|
1. ↑ tubular reabsorption of Na+ and K+ secretion
2. ↑ exchange transport of Na+ and H+ 3. ↑ ECF volume |
|
|
List actions of glucocorticoids. (5)
|
1. ↑ plasma [glucose], [protein], [fat]
2. ↓ plasma [Ca++] by inhibiting Ca absorption in the intestine and increasing Ca excretion 3. inhibits GH 4. anti-inflammatory 5. changes in the formed elements of blood (1) ↑ Hb, RBC content, neturopihls (2) ↓ lymphocytes, monocytes, eosinophils |
|
|
List pharmacokinetics of corticosteroids.
|
1. > 90% cortisol bound to CBG (cortisol binding globulin) and albumin
2. 80% cortisol metabolism in liver and excreted in the urine 3. Tissue t1/2 = 8-12 hrs 4. Greatest potential for drug interactions occurs when corticosteroids are paired with drugs that induce hepatic enzymes 5. Aldosterone weakly bound to albumin – shorter duration of action |
|
|
What is the advantage of using synthetic corticosteroids over natural ones?
|
1. effective in producing separation of glucocorticoid and mineralocorticoid activities
eg. inflammatory potency can be obtaine din absence of Na++ retaining effects |
|
|
Name 2 synthetic glucocorticoids that have high antiinflammatory potetncy but low Na++ retaining activities.
|
Prednisone
Dexamethasone |
|
|
Name 3 corticosteroid synthesis inhibitors.
MOA Indications? |
Metyrapone, Aminoglutethimide, Ketoconazole
MOA - inhibits 11-beta hydroxylase; indicated for Cushing's disease |
|
|
Name 3 corticosteroid synthesis inhibitors.
MOA Indications? |

Metyrapone, Aminoglutethimide, Ketoconazole
MOA - inhibits 11-beta hydroxylase; indicated for Cushing's disease |
|
|
What is Addison's disease?
Is this primary or secondary? How do you treat it? |
adrenocortical insufficiency
primary replace both glucocorticoids and mineralocorticoids |
|
|
What are some symptoms due to deficiency of mineralocorticoids? (6)
|
(1) Hypotension
(2) Hypoatremia (3) Lethargy (4) Easy fatigue (5) Vertigo (6) Syncope |
|
|
What are some symptoms due to dificiency of glucocorticoids? (8)
|
(2) Fatigue
(3) Weight loss (4) Hypoglycemia (5) Pigmentation increase (6) Irritability (7) Mental sluggishness (8) Increases sensitivity to taste |
|
|
Describe Cushing's syndrome.
Etiology (3)? symptoms? treatment? |
excessive secretion of adrenocorticoids (both mineralocorticoids and glucocorticoids)
etiologies 1. adrenal hyperplasia or tumor 2. ectopic ACTH- secreting neoplasma 3. pituitary tumor symptoms - hypertension, glucose intolerance, and obesity treat with 11-beta hydroxylase blocker (metyronpone, aminoglutethimide, or ketoconazole) |
|
|
What is Conn's syndrome?
etiology? symptoms? Treatment? |
excessive mineralocorticoids
adrenocortical adenoma symptoms - hypertension, hypokalemia, high plasma volume, alkalosis treatment - epleronone(aldosterone antagonist) prior to surgery |
|
|
How do you diagnose b/t primary and secondary adrenocortical insufficiency?
|
low plasma ACTH - secondary
high plasma ACTH - primary |
|
|
How do you diagnose b/t primary and secondary etiology of Cushing's syndrome?
|
low plasma ACTH - primary
high plasma ACTH - secondary |
|
|
What is the primary use of steroidal drugs?
High-dose suppressive are glucocorticoid used is for? low-dose suppressive are glucocorticoids used for? |
primary - replacement therapy for adrenocortical insufficiency
high-dose GC - reserved for lifte-threatening conditions 1. collagen diseases - e.g. SLE 2. Rheumatic carditis 3. organ transplantation low-dose GC - non-endocrine diseases for palliative properties but not curative |
|
|
What is the advantage of alternate-day therapy (glucocorticoid) for chronic treatment?
|
preferred because this lessens the suppression of hypo-ant. pit-adrenal axis.
The axis suppression is more depedent on duration of depressed ACTH than on degrees of depression |
|
|
What is the right protocol of withdrawing from chronic glucocorticoid therapy?
|
Gradual dose reduction to avoid life-threatening adrenal insufficiency.
|
|
|
List glucocorticoid toxicities. (6)
|
1. Osteoporosis
2. peptic ulcer 3. growth inhibition in children cannot be overcome with GH 4. behavioral disturbances 5. increased susceptibility to infection 6. glycosuria 7. myopathy |
|
|
List mineralocorticoid toxicities. (4)
|
1. hypoalkemia --> muscular paralysis
2. hypertension 3. edema 4. alkalosis |
|
|
What time of the day is the testosterone level highest?
|
Early AM
follows the Circadian rhythm |
|
|
What is the primary control of LH release? FSH release? (in male)
|
Testosterone negative-feedbacks to ant. pit. --> inhibits release of LH
Inhibin negatively feedbacks to Ant. pit --> inhibit release of FSH Activin stimulates FSH release |
|
|
Describe pharmacokinetical properties of natural testosterone.
|
short half-life of 10-20 mins
metabolized in liver and renal excretion |
|
|
How is synthetic testosterone different from natural ones?
Indication? |
Esterification of testosterone --. slower absorption and metabolism
Indicated for treatment for androgen-deficient males for development or maintenance of secondary sex characteristics |
|
|
Name 2 orally effective testosterone.
Contraindicated for? |
methyltestosterone and flouxymesterone.
contraindicated for prolonged use due to hepatotoxicity |
|
|
List 3 androgen side effects.
|
1. virilism, especially in in prepuberal children, women, and older men.
2. liver dysfunction, jaundice, and liver cancer 3. premature eiphyseal closure in children. |
|
|
List 2 therapeutic uses of androgens
|
1. constitutional delay of growth
2. hypogonadism |
|
|
Name 3 antiandrogens.
MOA for each |
Finasteride - 5-alpha reductase inhibitor
Flutamide - androgen receptor antagonist Cyproterone - androgen receptor antagonist |
|
|
Finasteride
MOA Indication. |
antiandrogen
5alpha reductase inhibitor --> blocks conversion in target tissue from T to more active form indicated in benign prostatic hyperplasia |
|
|
Flutamide
MOA Indication disadvantage |
Androgen receptor antagonist
indicated as adjunct therapy for prostatic carcinoma weak affinity for the receptor block T feedback to pituitary --> LH secretion goes up --> endogenous T goes up --> competes with flutamide |
|
|
Cyproterone acetate
MOA Indication side effects |
potent antiandrogens
androge receptor antagonist; alos has progestin/estrogen activity --> suppress both LH and FSH effective in treating androgenic effects of precocious puberty and prostatic carcinoma causes atrophy of androgen-responsive organs and pituitary changes typical of castration |
|
|
How does continuous administration of GnRH agonist suppress androgen production?
often used with what other drug? why? |
Desensitize GnRH receptors in AP --> decreased stimulation of LH/FSH
Flutamide b/c less endogenous testosterone for competition for androgen receptor |
|
|
What cell/tissue produces estrogen?
Regulation? |
Produced in granulosa cells (follicle), corpora lutea, and placenta
FSH stimulation needed for secretion of estrogen. Estrogen and and inhibin - negative feedback to ant. pit/hypothalamus activin - positive modulation of FSH |
|
|
List some actions of estrogen. (8)
|
1. cell growth of sexual organs
2. stimulate uterine growth (endometrium) 3. decrease PTH effect on osteoclasts 4. increase metabolic effect slightly 5. fat deposition 6. Decrease LDL and increase HDL 7. increase tubular Na+ reabsorption 8. neurotrophic and neuroprotective |
|
|
What tissues make/secrete natural progestin?
Regulation? |
From corpora lutea and placenta
LH stimulates the release of progestin. Progestin - negative feedback to ant. pit./ hypothalamus |
|
|
List some actions of progestin
|
1. promote secretory changes in endometrium in prep for implantation
2. Promote lobular and alveolar development of breasts 3. Increase Na+ excretion and water; competes with aldosterone, but progestins’ effects are weak 4. Increase insulin levels, insulin response to glucose 5. CNS effects – depressant and hypnotic effects on neuronal activity |
|
|
List 4 different phamacological forms of estrogen. Briefly explain each.
|
1. natural estrogen - rapid metabolism in liver; can't be used therapeutically
2. Estrogen ester - slow onset and decline of action 3. Conjugated estrogen - orally effective but low potency 4. alkylated estrogens - rally effective, longer duration of action (e.g. ethinyl estradiol) |
|
|
Name 4 orally effective progestins.
|
Norgestrel, norethindrone, ethynodiol, norethynodrel
|
|
|
List 6 clinical indications for uses of estrogen/progestin.
|
1. Amenorrhea
2. primary hypogodnadism 3. Metastatic endometrial carcinoma - progestin therapy 4. diagnostic test - progestin can be used to assess estrogen secretion 5. menopausal changes 6. osteroporosis after menopause |
|
|
What are SERMs? List 3 examples
|
Selective estrogen receptor modulators - mixed estrogen agonist and antagonist properties
tamoxifen, clomiphene, raloxifene |
|
|
Aromatase inhibitors are used for what? List 2 examples of the drug
|
used in treating breast cancer in postmenopausal women.
anastrozole and exemstane |
|
|
What 2 antiestrogens are used for breast cancer?
Which one is preferred, why? |
Tamoxifen and raloxifene
Raloxifene is preferred b/c fewer side effects |
|
|
Mifepristone
MOA? Side effects? |
progesterone antagonist
controversy over potential use of induced abortions |
|
|
What is Ganirelix?
|
GnRH antagonist
|
|
|
What is the indication for fertility drug therapy?
Name 3 and briefly explain them. |
Indication - failiure of ovulation
1. clomeiphene - estrogen antagonist at hypothalamus and anterior pit 2.Urofollitropins -FSH 3. Gonadorelin - GnRH; used in conjunction with HcG |
|
|
What are 2 main components of oral contraceptives?
Rationale for each component? |
Estrogen - FSH inhibition --> ovulation and follicular growth inhibited
Progestin - protective against estrogen's ability to cause endometrial cancer |
|
|
Name 2 synthetic estrogens.
List 3 phamacokinetic properties. |
Ethinyl estradiol and mestranol
1. Rapid and complete absorption from GI 2. Binds to sex-hormone-binding globulin or albumin 3. Slower hepatic metabolism |
|
|
Which synthetic progestin has an antagonist property toward estrogen? additive property?
|
Antagonistic - norgesterel
additive - norethynodrel |
|
|
What drug formulation was adapted in the combination OC therapy to protect the endometrial lining with minimal amt of progestin taken?
|
biphasic and triphasic formulation.
|
|
|
What are minipills?
disadvantage? indication? |
Oral contraceptive with progestin only.
Less effective and higher side effects ovulation is not consistently suppressed indicated for women who should avoid estrogen (hypertension and migraine) |
|
|
What are post-coital contraceptives?
Indication? MOA? Side effects? |
high doses of estrogen or progestin.
Designed to be effective after fertilization has occurred interferes with tubal transport, alters endometrial environment side effects - nausea and vomiting |
|
|
What is the main ingredient of "plan B?"
|
OTC progestin only
|
|
|
What should be the main therapeutic principles in prescribing OCs?
|
1. Preparation with smallest quantity of hormone consistent with efficacy and tolerable side effects is preferred
2. Adjustment of medication should be based on restoring estrogen-progestin balance according to symptoms observed with previous preparations |
|
|
List 4 ways OCs can be interfered with other drugs.
|
1. competition for pathways for hepatic metabolism
2. hepatic enzyme induction 3. competition for plasma protein binding 4. alter results of some common lab test |
|
|
What 2 hormones interacts with oxytocin? how?
|
Estrogen - stimulate oxytocin and increases uterine responsiveness
Progestin - inhibits oxytocin and decreases uterine responsiveness |
|
|
How does Estrogen/progestin ratio change towards the parturition?
|
Increases.
|
|
|
Name 3 uterine stimulants.
|
1. Oxytocin
2. Prostaglandin (Dinoprostone/PGE2) 3. Ergot alkaloids (Ergonovine) |
|
|
Name 3 uterine relaxants.
|
1. Beta-2 agonists (Ritodrine, Terbutaline)
2. Naproxene (NSAID) 3. MgSO4 |
|
|
Oxytocin
Indication Route of administration Contraindication |
Uterine stimulant
Indications 1. drug of choice for induction of labor at term 2. post-partum use to prevent hemorrhage and correct hypotonicity IV administration (short half-life) contraindications 1. not used in a slow labor 2. not for patients with complications 3. not for patients with previous uterine surgery |
|
|
Dinoprostone (PGE2)
Route of administration Indications side effects |
Uterine stimulant (cervical rippening agent)
Intravaginal is most promising Indicated to induce labor side effects - GI smooth muscle contraction and unphysiological contractions |
|
|
Ergot alkaloids (Ergonovine)
route of administration Indications contraindications side effects |
Uterine stimulant
Oral or IM Used post-partum to prevent bleeding and maintain uterine muscle tone Not used to induce labor contraindicated in hypertensive patients side effects - worse after IV; CV and GI signs |
|
|
Ritodrine (Terbutaline)
route of admin Contraindication |
beta2 agonist; Uterine relaxant
IV most effective; oral after discharge contraindicated if the continuation of pregnancy is greater hazard |
|
|
Naproxen
MOA Advantage adverse effects |
uterine relaxant
NSAID; blocks synthesis of prostaglandin --> interruption or slowing of labor longer duration of action - 14 hrs GI irritation and renal/hepatic toxicity |
|
|
MgSO4
MOA Contraindication preferred in? |
Uterine relaxant
direct effect on myometrium and uncouples contraction and excitation contraindicated in cardiac, renal disease preferred over beta-2 agonists for patients with diabetes, hypertension, or hyperthyroidism |
|
|
Name a GH release stimulant and 2 inhibtors.
|
GHRH - stimulant
Somatostatin and IGF - inhibitors |
|
|
What is the role of IGF-1?
|
Insulin-like growth factor 1
Principal mediator of GH action. Mediates anabolic effects of GH and cellular processes of bone growth. |
|
|
List physiological actions of GH. (4)
|
1. Stimulate organ and tissue growth, esp. long bones.
2. stimulate AA uptake and protein synthesis 3. longer-term delayed effect - decreases glucose utilization, increases lipolysis and fatty acid oxidation 4. net positive effect on plasma Ca+2 levels |
|
|
What is the recommended treatment for GH deficiency?
If elevated GH does not induce changes? |
Synthetic GH (somatrem and somatropin)
Mecasemin (IGF-1) should be used if elevated GH does not induce changes |
|
|
Name 2 drug therapies recommended for acromegaly. what are they?
|
Bromocriptine - dopamine agonist
Octreotide - somatostatin analogue |
|
|
Actions of PTH? how?
|
elevates serum Ca+2, reduces serum PO4-3
dual effect: increased bone resorption, turnover; decreased activity of osteoblasts but increased activity of osteoclasts renal tubular reabsorption: lose PO4-3, save Ca+2 |
|
|
Actions of 1,25(OH)2D? how?
|
elevates serum Ca+2 and PO4-3
enhances intestinal Ca transport and bone resorption |
|
|
Actions of calcitonin?
Are the actions primary or secondary to PTH? |
secondary effect to transiently decrease bone resorption, plasma Ca+2
|
|
|
List 2 etiologies of hypocalcemic states. How are they different?
|
1. Hypoparathyroidism - decrease plasma Ca++
2. Vitamin D deficiency - plasma Ca++ near normal but plasma PO4 is low |
|
|
List 2 ways to treat hypocalcemia.
|
Calcium carbonate (oral, IV, Im)
Calcitriol (activated vitD) - usually combined with Ca++ supplement |
|
|
List 2 etiolgies of hypercalcemic state.
|
1. Primary hyperparathyroidism
2. hypervitaminosis D |
|
|
List 4 ways to treat hypercalcemia.
Indications for each? |
1. Loop diuretic (furosemide) - only for acute hypercalcemia
2. Glucocorticoids (prednisone) - for chronic conditoin 3. Calcitonin and alendronate therapy - used together for Paget's disease Alendronate - retards formation and dissolution of bone crystal |
|
|
4 Antiresorptive agents to treat osteoporosis.
|
1. Raloxifene (SERM) - agonist in bone and antagonist in uterus/breast
2. Aledronate (bisphosphnates) - retards bone crystal changes 3. Cyclic estrogen/progestin with Ca supplement 4. RANK-L inhibitors - block 1,25(OH)2D resorptive effects |
|
|
What is the rationale for using PTH for osteoporosis?
|
Low dose of PTH induces osteoblastic activity.
|
|
|
List 5 GI hormones that can amplify insulin secretion.
|
1. Glucagon-like peptide (GLP-1)
2. Gastric inhibitory peptide 3. gastrin 4. secretin 5. cholecystokinin (CCK) |
|
|
What are 2 major inhibitors of insulin secretion?
|
1. epinephrine (alpha adrenergic receptor)
2. beta adrenergic antagonists |
|
|
What amino acid amplify insulin secretion?
|
Arginine
|
|
|
Describe the mechanism of insulin release.
|
Increase in intracellular [glucose] in the beta cell --> more ATP --> close K+ channels --> depolarization --> Ca influx --> exocytosis of preformed insulin granules
|
|
|
Describe the paracrine control of insulin
|
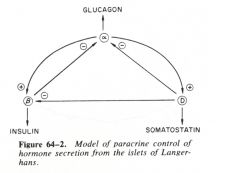
Glucagon stimulate insulin release
and somatostatin inhibits insulin release. |
|
|
How are the insulin preparations classified?
What is the route of administration of insulin preps? why? |
based on onset/duration of action; ultra-fast, fast, intermediate, and long-acting.
IV administration b/c rapidly metabolized |
|
|
List six insulin agents based on their speed of onset of action.
|
1. insulin lispro - ultra-fast
2. regular insulin - fast; only for emergency of hyperglycemia 3. isophane insulin (NPH) - intermediate acting 4. insulin zinc - intermediate acting 5. extended insulin zinc (ultralente) - slow acting 6. Glargine - slow acting |
|
|
What are the symptoms of insulin overdose?
|
Hypoglycemia
Symptoms are similar to that of high levels of epinephrine (counter hormone to insulin) Hunger, sweating, palpitations, most, pale skin, hypothermia |
|
|
How would you have a consistent control over blood glucose when on insulin regimens?
|
Combination insulin regimens using fast and long acting preparations
Portable pen injectors and pump for stricter regulation of blood glucose |
|
|
What are 3 complications of diabetes mellitus.
|
insulin resistance
diabetic ketoacidosis hyperosmolar coma |
|
|
Sulfonylurea
MOA Pharmacokinetcis indications adverse effects |
MOA - blocks K+ channels on the beta cells and induces insulin release; also bind to PPAR-gamma
PK - rapid and complete absorption; bind to plasma proteins; liver metabolism, renal excretion Indications for type II DM patients with adequate kidney and liver function Adverse effects - GI disturbances, water retention and hyponatremia |
|
|
What is tolbutamide? Indication?
|
sulfonylurea
Indicated for elderly patients b/c of less potency |
|
|
Meglitinides (glinides)
What is it? MOA? |
Insulin secretagogue
bind to PPAR gamma --> enhance beta cell response |
|
|
D-Phenylalanin derivatives
What is it? MOA? |
insulin secretagogue
close beta cell K+ channels --> release of insulin |
|
|
Thiazolidinediones (pioglitazone)
What is it? MOA? |
insulin sensitizer
reduce phosphorylation of PPAR-gamma receptor --> enhance target cell response |
|
|
Biguanides
MOA Advantage over other drugs Side effects |
Oral hypoglycemic (aka Metformin)
Enhance glucose utilization via lowering blood lipids inhibit mTOR through a GTPase no risk of hypoglycemia and weight gain; diminished CV risks Notable GI side effects |
|
|
alpha-glucosidase inhibitors
what is it MOA |
Oral hypoglycemic
slow glucose absorption via enzyme inhibition (insulin-sparing) |
|
|
Gliptins (Sitagliptin)
What is it? MOA Indications? |
Oral hypoglycemic
increase GLP-1 and GIP level --> increases insulin and decreases glucagon release useful as adjuncts to sulfonyurea/metformin therapy |

