![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
136 Cards in this Set
- Front
- Back
|
What kind of disease is Diabetes Mellitus?
|
Disease of metabolism - affects lipids, carbohydrates, and protein metabolism
|
|
|
What hormones control metabolism from fed to fasting/exercise states? Source?
|
Endocrine Pancreas:
- Insulin - Glucagon - Somatostatin |
|
|
How do you diagnose Diabetes Mellitus?
|
Any of the following:
- HbA1c ≥ 6.5% - Fasting plasma glucose ≥ 126 mg/dL - 2 hour plasma glucose during 75g oral glucose tolerance test ≥ 200 mg/dL - Random glucose ≥ 200 mg/dL |
|
|
How do you diagnose Pre-Diabetes Mellitus?
|
One of the following:
- Fasting plasma glucose = 100-125 mg/dL - 2 hr plasma glucose after 75g oral glucose tolerance test = 140-199 mg/dL - HbA1c = 5.7 - 6.4% |
|
|
How high does HbA1c have to be to make a diagnosis of Diabetes Mellitus? Pre-Diabetes?
|
- DM: ≥ 6.5%
- Pre-DM: 5.7 - 6.4% |
|
|
How high does the fasting plasma glucose have to be to make a diagnosis of Diabetes Mellitus? Pre-Diabetes?
|
- DM: ≥ 126 mg/dL
- Pre-DM: 100-125 mg/dL |
|
|
How high does a 2 hr plasma glucose during a 75g oral glucose tolerance test, have to be to make a diagnosis of Diabetes Mellitus? Pre-Diabetes?
|
- DM: ≥ 200 mg/dL
- Pre-DM: 140-199 mg/dL |
|
|
How high does a random serum glucose have to be to make a diagnosis of Diabetes Mellitus?
|
DM: ≥ 200 mg/dL
|
|
|
How did the values for diagnosing diabetes mellitus get determined?
|
Based on the level of glucose or HbA1c that is associated with development of complications, specifically retinopathy
|
|
|
What is HbA1c a measure of?
|
- Measure of glycosylated hemoglobin at the terminal valine of the beta chain
- Glucose is non-enzymatically attached to proteins - With higher concentration and longer the exposure to glucose, the more glycosylation occurs *So HbA1c is an integrated measure of glucose levels in the blood (correlates w/ complications of DM) |
|
|
What does a diagnosis of Pre-Diabetes mean?
|
Increased risk of developing Type 2 Diabetes Mellitus (at rate of 10% / year)
|
|
|
If you have been diagnosed with Pre-Diabetes, how much can you lower your chance of progressing to Type 2 DM?
|
- Usually progress at rate of 10%/year
- Can lower to about 5%/year w/ improved lifestyle (diet and exercise) |
|
|
What are the short-term complications of DM?
|
- Electrolyte abnormalities
- Fatigue - Poor wound healing - Impaired immune defenses - Prolonged hospital stay - Increased inpatient morbidity and mortality - Treatment related hypoglycemia |
|
|
What causes the fluid and electrolyte abnormalities in DM?
|
Hyperglycemia → Osmotic Diuresis → Fluid & Electrolyte abnormalities
|
|
|
What can cause the short-term complications of DM?
|
- Short-term elevation of glucose levels
- Osmotic diuresis - Direct toxic effect of glucose - Pathological intermediate metabolism |
|
|
What are the long-term complications of DM?
|
Microvascular:
- Retinopathy - Nephropathy - Neuropathy Macrovascular: - Coronary Artery Disease - Peripheral Vascular Disease |
|
|
What are the Microvascular long-term complications of DM?
|
- Retinopathy
- Nephropathy - Neuropathy |
|
|
What are the Macrovascular long-term complications of DM?
|
- Coronary Artery Disease
- Peripheral Vascular Disease |
|
|
How long does it take to get long-term complications of DM?
|
5 years or more
|
|
|
What can cause the long-term complications of DM?
|
- Chronic exposure to elevated glucose levels
- Higher the exposure, the quicker the complications develop - Microvascular complications related to damage to the small nutrient vessels - Macrovascular complications are d/t large vessel damage |
|
|
What can the microvascular and macrovascular complications lead to?
|
- Blindness
- Kidney failure - Amputations - Heart attacks - Strokes |
|
|
What is the pathogenesis of DM complications?
|
- Accumulation of advanced glycosylation end products
- Accumulation of sorbitol - Disruption of the hexosamine pathway - Disruption of the protein kinase C pathway - Activation of the poly (ADP-ribose) polymerase pathway - Increased oxidative stress |
|
|
What are the classification types of DM?
|
- Type 1
- Type 2 - Other specific types - Gestational |
|
|
What are the characteristics of Type 1 DM? How common?
|
- Autoimmune β-cell destruction of the Islet of Langerhans cells located in the pancreas, which leads to a lack of insulin
- 0.8% of the population |
|
|
What are the characteristics of Type 2 DM?
|
- Insulin secretion cannot mount a response adequate enough to overcome insulin resistance
- The insulin resistance is usually associated with obesity, particularly truncal adiposity, and poor physical fitness - 8% of the population. |
|
|
What can cause other specific types of DM besides Type 1 and 2?
|
- Genetic defects in β-cell insulin secretion
- Cystic Fibrosis - Alcoholic pancreas disease - Pancreatic cancer - Cushing's syndrome - Endocrinopathies - Drug- or chemical- induced - Other rare forms |
|
|
βWhat are the characteristics of Gestational DM?
|
- Insulin resistance with β-cell dysfunction
diabetes that develops during pregnancy - Pregnancy is an insulin resistance state: placenta secretes hormones which cause insulin resistance in the mother, so that nutrients can be directed to the growing fetus - In those with a predisposition, pregnancy can unmask abnormal glucose metabolism; sometimes, however, the immunological changes during pregnancy brings on type 1 diabetes |
|
|
What defects are there in T2DM?
|
- Insulin resistance
- Inadequate insulin secretion |
|
|
What are the implications of insulin resistance in T2DM?
|
- Inability to suppress glucose production from the liver and kidney during fasting
- Inability to appropriately take-up glucose by insulin responsive tissues (skeletal muscle, liver and fat) after eating. |
|
|
What is the natural history of plasma glucose in T2DM?
|
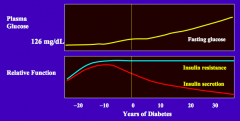
Gradually the fasting glucose increases (>126 mg/dL)
|
|
|
What is the natural history of insulin secretion in T2DM?
|
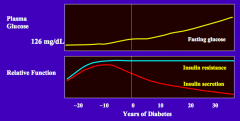
- The pancreas increases insulin secretion to maintain good glycemic control in the face of the insulin resistance
- Over time, insulin secretion wanes and hyperglycemia and diabetes mellitus develops |
|
|
What is the natural history of insulin resistance in T2DM?
|
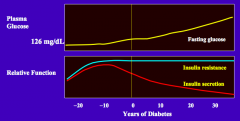
Initially, due to genetic predisposition and poor lifestyle, patient develop insulin resistance
|
|
|
How are insulin resistance and insulin secretion affected by an improved lifestyle (healthy diet and increased exercise)?
|
Both insulin resistance and insulin secretion can improve
|
|
|
What are the markers of improved lifestyle?
|
- Improved physical fitness
- Decreased fat mass - Increased lean mass - Decreased truncal adiposity - Decreased total weight |
|
|
What are the types of medications used for T2DM?
|
- Insulin
- Sulfonylurea - Meglitinide - Biguanide - Alpha-Glucosidase inhibitors - Thiazolidinediones - Incretin analogs - DPP-4 inhibitors - Sodium glucose co-transporter 2 inhibitor |
|
|
Which drug has the following mechanism:
- Binds to insulin receptor to increase glucose uptake by insulin responsive tissues |
Insulin
|
|
|
Which drug has the following mechanism:
- Binds to receptor that regulates K+-channel to stimulate insulin release (longer half-life) |
Sulfonylurea: eg, Glimepiride
|
|
|
Which drug has the following mechanism:
- Increases insulin secretion, also by binding to K+ channel but w/ a short half-life |
Meglitinide: eg, Repaglinide and Nateglinide
|
|
|
Which drug has the following mechanism:
- Decreases systemic glucose output |
Biguanide: eg, Metformin and Phenformin
|
|
|
Which drug has the following mechanism:
- Delays digestion of complex carbohydrates |
Alpha-glucosidase inhibitor: eg, Acarbose
|
|
|
Which drug has the following mechanism:
- Binds to PPAR receptors and appears to sensitize insulin responsive tissues to insulin |
Thiazolidinediones (Glitazones): eg, Rosiglitazone and Pioglitazone
|
|
|
Which drug has the following mechanism:
- Binds to ____ receptors to increase prandial insulin secretion and delay gastric emptying |
Incretin analogs (bind incretin receptors): eg, Exenatide and Liraglutide
|
|
|
Which drug has the following mechanism:
- Inhibits the break-down of incretins |
DPP-4 inhibitor: eg, Stiagliptin
|
|
|
Which drug has the following mechanism:
- Inhibits glucose reabsorption of filtered glucose in the kidney |
Sodium glucose co-transporter 2 inhibitor: eg, Canaglifozin
|
|
|
What is the most powerful agent to lower glucose?
|
Insulin
|
|
|
Insulin:
- Mechanism - Efficacy - Side effects - Other |
- Mechanism: binds to insulin receptor to increase glucose uptake by insulin responsive tissues
- Efficacy: most powerful agent to lower glucose - Side effects: can cause hypoglycemia - Other: requires good self-management habits |
|
|
Sulfonylurea:
- Drug names - Mechanism - Efficacy - Side effects |
- Drug names: Glimepiride
- Mechanism: binds to receptor that regulates K+ channel to stimulate insulin release - Efficacy: inexpensive - Side effects: can cause hypoglycemia |
|
|
Meglitinide:
- Drug names - Mechanism - Efficacy - Side effects - Other |
- Drug names: Repaglinide and Mateglinide
- Mechanism: increases insulin secretion by binding to K+ channel (short half-life) - Efficacy: can be used in chronic renal disease - Side effects: can cause hypoglycemia |
|
|
Biguanide:
- Drug names - Mechanism - Efficacy - Side effects - Other |
- Drug names: Metformin and Phenformin
- Mechanism: decreases systemic glucose output - Efficacy: does not cause hypoglycemia - Side effects: can cause lactic acidosis - Other: can not use in chronic renal disease, pulmonary disease, liver disease, or heart disease |
|
|
Alpha-Glucosidase Inhibitor:
- Drug names - Mechanism - Side effects |
- Drug names: Acarbose
- Mechanism: delays digestion of complex carbohydrates - Side effects: excess flatus |
|
|
Thiazolidinediones:
- Drug names - Mechanism - Efficacy - Side effects - Other |
- Drug names: Glitazones = Rosiglitazone and Pioglitazone
- Mechanism: binds to PPAR receptors and appears to sensitize insulin responsive tissues to insulin - Side effects: fluid overload that can cause heart failure - Other: associated w/ acute coronary syndrome and bladder cancer |
|
|
Incretin Analogs:
- Drug names - Mechanism - Side effects - Other |
- Drug names: Exenatide and Liraglutide
- Mechanism: bind to incretin receptors to increase prandial insulin secretion and delay gastric emptying - Side effects: nausea, pancreatitis and pancreatic cancer - Other: injections |
|
|
DPP-4 Inhibitor:
- Drug names - Mechanism - Side effects |
- Drug names: Stiagliptin
- Mechanism: inhibits the breakdown of incretins - Side effects: nausea, pancreatitis, and pancreatic cancer |
|
|
Sodium-Glucose Co-transporter 2 Inhibitor:
- Drug names - Mechanism - Efficacy - Side effects |
- Drug names: Canagliflozin
- Mechanism: inhibits glucose reabsorption of filtered glucose in the kidney - Efficacy: limited clinical experience - Side effects: increased UTIs |
|
|
Case 1: 64 yo female has FHx of T2DM. She does little exercise. She has truncal adiposity w/ BMI of 34. BP is 126/72 and HR of 82. Her feet show no lesions, w/ good pulses and normal vibratory sensation.
You want to test for the presence of diabetes mellitus. Which test results is consistent with diabetes mellitus? a) Fasting glucose of 88 mg/dL b) 1 hour glucose level during a glucose tolerance test of 205 mg/dL c) 2 hour glucose level during a glucose tolerance test of 148 mg/dL d) Random glucose of 180 mg/dL e) Hemoglobin A1c of 7.8% |
HbA1c of 7.8% (e)
|
|
|
Case 1: 64 yo female has FHx of T2DM. She does little exercise. She has truncal adiposity w/ BMI of 34. BP is 126/72 and HR of 82. Her feet show no lesions, w/ good pulses and normal vibratory sensation.
She is found to have diabetes mellitus with a HA1c of 7.8%. You diagnose her with T2DM. She is sent to the nurse educator and dietitian. What will the nurse educator teach her on the pathophysiology of her diabetes mellitus? |
She does not make enough insulin to overcome her insulin resistance
|
|
|
Case 1: 64 yo female has FHx of T2DM. She does little exercise. She has truncal adiposity w/ BMI of 34. BP is 126/72 and HR of 82. Her feet show no lesions, w/ good pulses and normal vibratory sensation.
After 3 months of diet and exercise, following the regimen suggested by the nurse educator and dietitian, her A1c is still above goal. You discuss further treatment options. She has an irregular timing to her meals and is concerned about hypoglycemia. Which treatment regimen will you suggest? |
Metformin
|
|
|
Case 2: 18 yo college freshman who complains of very stinky feet. He admits to being thirsty frequently, urinating more. He urinates 3x/night, is fatigued, is not active, and has gained weight since starting college in the fall. His father has T2DM. He is obese w/ BMI of 35. He has acanthosis nigracans on his neck. He has severe tinea pedis. You suspect that he has DM - his HbA1c is 12.5%
What did NOT contribute to his development of diabetes mellitus? a) Inactive lifestyle b) Obesity c) Genetic predisposition d) Absolute insulin deficiency e) Relative insulin deficiency |
Absolute insulin deficiency - this is associated w/ T1DM
|
|
|
Case 2: 18 yo college freshman who complains of very stinky feet. He admits to being thirsty frequently, urinating more. He urinates 3x/night, is fatigued, is not active, and has gained weight since starting college in the fall. His father has T2DM. He is obese w/ BMI of 35. He has acanthosis nigracans on his neck. He has severe tinea pedis. You suspect that he has DM - his HbA1c is 12.5%
So, he has newly diagnosed T2DM. There are many short-term complications of DM. Which of the following is our college student NOT at risk for at this time? a) Fatigue b) Hypoglycemia c) Increased infections d) Poor wound healing |
Hypoglycemia
|
|
|
Case 2: 18 yo college freshman who complains of very stinky feet. He admits to being thirsty frequently, urinating more. He urinates 3x/night, is fatigued, is not active, and has gained weight since starting college in the fall. His father has T2DM. He is obese w/ BMI of 35. He has acanthosis nigracans on his neck. He has severe tinea pedis. You suspect that he has DM - his HbA1c is 12.5%
To begin treatment, he meets with the nurse educator, who outlines the long-term complications of diabetes mellitus to the patient.People with poorly controlled diabetes mellitus are at increased risk for these complications EXCEPT? a) Leg amputations b) Cerebellar vascular accidents c) Myocardial infarctions d) Renal failure requiring a kidney transplant e) Osteoporosis |
Osteoporosis
|
|
|
Case 3: You are following up w/ a 64-yo patient w/ a 20 year history of T2DM. He also has dyslipidemia and HTN. His most recent HbA1c is 9.2%. He has complications of retinopathy, treated w/ laser surgery, coronary artery disease, treated w/ a stent, and polyneuropathy.
The elevated A1c is an example of which metabolic pathway that produces diabetes complications? a) Disruption of the hexosamine pathway b) Increased oxidative stress c) Accumulation of advanced glycosylation end products d) Accumulation of sorbitol |
Accumulation of advanced glycosylation end products
|
|
|
Case 3: You are following up w/ a 64-yo patient w/ a 20 year history of T2DM. He also has dyslipidemia and HTN. His most recent HbA1c is 9.2%. He has complications of retinopathy, treated w/ laser surgery, coronary artery disease, treated w/ a stent, and polyneuropathy.
The patient has many complications. Which other macrovascular complication clinical manifestation might you anticipate? a) Nephropathy b) Cancer c) Mononeuropathy d) Ischemic stroke |
- Ischemic Stroke is a MACRO-vascular complication
- Nephropathy and mononeuropathy are microvascular complications - Cancer is not a vascular complication but it is a possible complication of DM |
|
|
Case 3: You are following up w/ a 64-yo patient w/ a 20 year history of T2DM. He also has dyslipidemia and HTN. His most recent HbA1c is 9.2%. He has complications of retinopathy, treated w/ laser surgery, coronary artery disease, treated w/ a stent, and polyneuropathy.
The patient is above A1c goal. He is currently on 3 oral medications – glimepiride, metformin and sitaglipitin. You are going to adjust his medications. Which medication is most likely to lower his A1c to goal? |
Insulin
|
|
|
What is the most powerful agent to lower HbA1c?
|
Insulin
|
|
|
What is a first-line agent for treating T2DM? Benefits?
|
Metformin (and does not cause hypoglycemia)
|
|
|
What initiates the pathophysiology of T1DM in children?
|
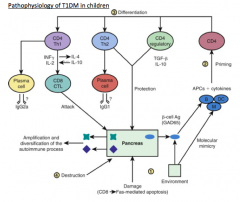
An insult to the pancreas leads to the release of β-cell antigens (GAD65), which are taken up by APCs and the epitopes presented to the CD4 T cells
|
|
|
What antigens are released when the pancreas incurs an insult (pathophysiology of T1DM)?
|
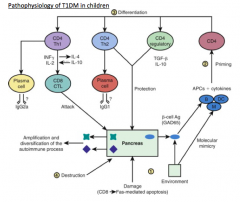
β-cell antigens (GAD65)
|
|
|
What dictates the differentiation of auto-reactive T cells (pathophysiology of T1DM)? Outcomes?
|
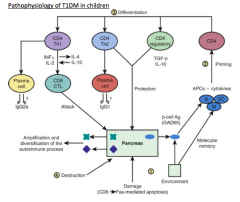
- Type and stages of activation of APCs as well as the cytokine environment in which the CD4 T cell priming takes place, dictates the differentiation
- Can differentiate into diabetogenic T helper-1 (Th1) cells, Th2 cells, or antigen-specific regulatory T cells (Tregs) |
|
|
What are the results of a predominant Th1 auto-immune response (pathophysiology of T1DM)?
|
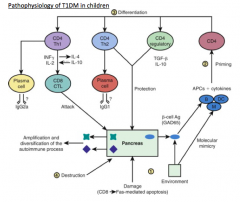
Recruitment and differentiation of cytotoxic CD8 cells, which attack the pancreatic β cells, leading to a massive release of β-cell antigens, epitope spreading, and destruction of the pancreatic islets
|
|
|
What is the natural history of T1DM?
|
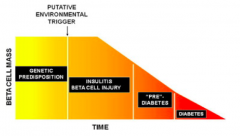
- Genetic predisposition → (putative environmental trigger) →
- Insulitis and β-cell injury → - Pre-diabetes → - Diabetes |
|
|
What are the common electrolyte changes in untreated T1DM?
|
- Hyponatremia
- Hyperkalemia or Hypokalemia - Low CO2 / bicarbonate - Phosphate (negative balance) |
|
|
What happens to the sodium balance in untreated T1DM?
|
OSMOTIC DILUTION OF PLASMA Na+ = HYPONATREMIA:
- Hyperglycemia will increase the plasma osmolality, resulting in osmotic movement out of the cells which lowers the serum sodium by dilution - Counteracted to variable degree by glucosuria-induced osmotic diuresis → water loss in excess of Na+ and K+ - Thirst (stimulated by hyperosmolality) may also contribute to hyponatremia |
|
|
What happens to the potassium balance in untreated T1DM?
|
Hypokalemia or Hyperkalemia (inconsistent finding):
- Osmotic diuresis and increased ketoacid excretion promote urinary K+ loss - Vomiting and diarrhea, if present, increase GI K+ loss - Insulin deficiency, which impairs K+ entry into cells, and hyperosmolality, which pulls K+ out of cells, tend to raise serum K+ - Serum K+ at time of presentation can be normal, increased, or decreased - Therapy w/ insulin and fluids will LOWER K+ concentration (need to monitor) |
|
|
What happens to the bicarbonate balance in untreated T1DM?
|
Low "CO2" (i.e., bicarbonate):
- Insulin deficiency and increased plasma conc. of glucagon, cortisol, and epi increase glucose production, lipolyis, and ketogenesis - These collectively contribute to the development of both Hyperglycemia and Ketoacidosis |
|
|
What happens to the phosphate balance in untreated T1DM?
|
DECREASED PHOSPHATE INTAKE AND PHOSPHATURIA:
- Children w/ DKA typically are in negative phosphate balance b/c of decreased phosphate intake and phosphaturia - Caused by glucosuria-induced osmotic diuresis - At presentation, despite the presence of phosphate depletion, serum phosphate may be normal or even high b/c of insulin deficiency and metabolic acidosis (which shifts phosphate out of cells) - True state of phosphate balance is unmasked after tx w/ insulin |
|
|
How does the number of male and female children compare for D1TM?
|
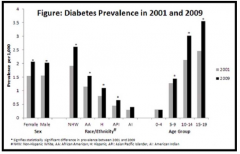
They are equal
|
|
|
Which ethnicities are more greatly affected by D1TM?
|
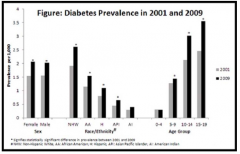
Non-Hispanic White > African Americans > Hispanics > Asian Pacific Islanders > Native Americans
|
|
|
What is the peak age for diagnosis of D1TM?
|
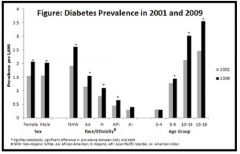
Between 10-19 years
|
|
|
What are the symptoms of new onset T1DM in children?
|
- Increased thirst (polydipsia)
- Increased urination (polyuria) - Bed-wetting (enuresis) - Weight loss - Increased hunger (polyphagia) - Fatigue |
|
|
How do you diagnose T1DM in children?
|
- Fasting blood sugar >126 mg/dL x2
- Random blood sugar >200 mg/dL - Oral glucose tolerance test >200 mg/dL at 2 hrs |
|
|
What is the procedure for the glucose tolerance test? What do you expect?
|
- Ingest a glucose load (75g)
- Plasma glucose rises slowly, reflecting the intestinal uptake of glucose - In response, pancreatic β cells secrete insulin - Plasma insulin rises sharply |
|
|
What is the response to an oral glucose tolerance test in a diabetic patient?
|
- Same glucose load causes glucose to rise to a higher level and to remain there longer
- Insulin rises very little in response to the glucose challenge and cells are unable to transport glucose |
|
|
What is the response to an IV glucose tolerance test in a diabetic patient?
|
- Plasma glucose rises much more rapidly than it does w/ an oral glucose load
- Sensing a rapid rise in glucose, the β cells first secrete some of their stores of presynthesized insulin - Following acute phase, cells secrete both presynthesized and newly manufactured insulin in chronic phase - This lasts as long as the glucose challenge |
|
|
What is the biochemistry / pathogenesis of diabetic ketoacidosis?
|
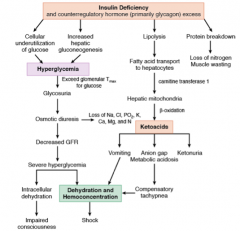
- Diabetic ketoacidosis occurs in the presence of absolute insulin deficiency
- This occurs in the setting of poor calorie consumption, prolonged poor glycemic control and often concomitant illness - The prolonged poor glycemic control further reduces the ability of the pancreas to secrete insulin, a principle called “glucose toxicity” |
|
|
In what setting does a diabetic have absolute insulin deficiency?
|
- Poor calorie consumption
- Prolonged poor glycemic control - Often concomitant illness - The prolonged poor glycemic control further reduces the ability of the pancreas to secrete insulin, a principle called "glucose toxicity" |
|
|
What is the body's response to ketoacidosis?
|
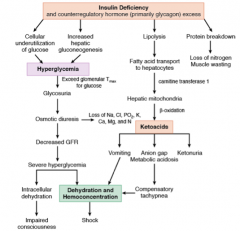
Compensatory hyperpnea (Kussmaul respiration):
- Body’s attempt to compensate for the metabolic acidosis by “blowing off” more CO2 |
|
|
What are the implications of insulin deficiency?
|
- Cellular underutilization of glucose AND
- Increased hepatic gluconeogenesis = Hyperglycemia - Lipolysis → FA transport to hepatocytes → hepatic mitochondria oxidate FA to ketoacids via β-oxidation Ketoacids |
|
|
What are the implications of ketoacidosis?
|
- Vomiting
- Anion gap / metabolic acidosis → compensatory tachypnea - Ketonuria - Vomiting and tachypnea → dehydration and hemoconcentration |
|
|
What are the short-acting insulin preparations (times of action onset)? Durations?
|
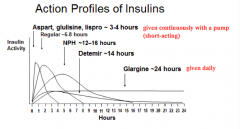
Effective duration: 3-4 hrs
- Aspart: <0.25 hr - Glulisine: <0.25 hr - Lispro: <0.25 hr Effective duration: 4-6 hrs - Regular: 0.5 - 1.0 hr |
|
|
What are the long-acting insulin preparations (times of action onset)? Durations?
|
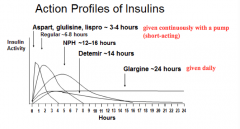
Effective duration: 12-16 hrs
- Detemir: 1-4 hrs Effective duration: 20-24 hrs - Glargine: 1-4 hrs Effective duration: 10-16 hrs - NPH: 1-4 hrs |
|
|
Which forms of insulin are given in a pump continuously?
|
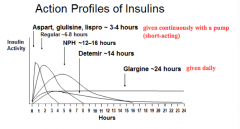
Short-acting preparations ~3-4 hrs:
- Aspart - Glulisine - Lispro |
|
|
Which form of insulin has the longest duration of action?
|
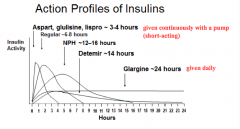
Glargine ~24 hrs
|
|
|
What are the mild effects of insulin overdose?
|
Hypoglycemia (blood sugar < 70 mg/dL), in response to increased sympathetic tone →
- Shakiness - Headaches - Dizziness - Sweatiness - Tachycardia - Hunger - Fatigue |
|
|
How do you treat insulin overdose / mild hypoglycemia?
|
Oral fast-acting carbohydrates (juice, candy) and rechecking the blood sugar in 15 minutes until rising
|
|
|
What are the symptoms of moderate hypoglycemia?
|
Neuroglycopenic symptoms:
- Confusion - Combativeness - Poor coordination - Slurred speech |
|
|
How do you treat moderate hypoglycemia?
|
Fast-acting carbohydrates (such as frosting or sugar-gel, given orally or in the buccal mucosa)
|
|
|
What are the symptoms of severe hypoglycemia?
|
- Stupor
- Seizure - Coma |
|
|
How do you treat severe hypoglycemia?
|
Emergency care w/ glucagon injections (after EMS is called)
|
|
|
Which foods can cause a more gradual rise and fall in blood sugars? Why?
|
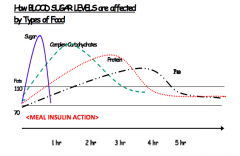
Foods high in fiber, protein, and fat (b/c they take longer to digest)
|
|
|
What foods do you eat to treat low blood sugars? What foods do you eat to sustain blood sugars over a longer period of time?
|
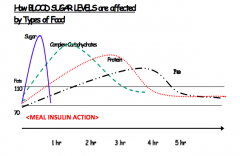
Treat low blood sugar:
- Quickly-digesting foods, such as juice and candy Sustain blood sugar (eg, overnight or before exercise) - Add protein or healthy fats to slow digestion |
|
|
Case 4: Bobby is a 7-yo boy who presents w/ concerns of fatigue and increased thirst. Mother reports he has less energy and unable to keep up w/ peers. Appetite has been good. He has had a recent growth spurt but his weight is unchanged. He has had 2 episodes of bed-wetting over last 2 weeks despite being potty-trained at 4. Mom thinks clothes are getting looser.
You suspect T1DM. Understanding the pathophysiology of the disease, which would be most consistent with this diagnosis? a) Pancreatic alpha cells are systematically destroyed b) Bobby ate too many Christmas cookies c) TH1 and CD8-mediated destruction of beta cells d) Insulin resistance led to inability to respond to carbohydrates e) His bedwetting is due to vasopressin deficiency |
Th1 and CD8 mediated destruction of beta cells
|
|
Case 4: Bobby is a 7-yo boy who presents w/ concerns of fatigue and increased thirst. Mother reports he has less energy and unable to keep up w/ peers. Appetite has been good. He has had a recent growth spurt but his weight is unchanged. He has had 2 episodes of bed-wetting over last 2 weeks despite being potty-trained at 4. Mom thinks clothes are getting looser.
What is the best course of treatment for Bobby? |
Glargine and lispro insulin
|
|
|
Case 4: Bobby is a 7-yo boy who presents w/ concerns of fatigue and increased thirst. Mother reports he has less energy and unable to keep up w/ peers. Appetite has been good. He has had a recent growth spurt but his weight is unchanged. He has had 2 episodes of bed-wetting over last 2 weeks despite being potty-trained at 4. Mom thinks clothes are getting looser.
Which of the following would be a better choice for a bedtime snack for 30g? a) 2 pieces of white toast b) 8oz orange juice c) 10 Triscuit crackers with cheese slices d) 30 Skittles e) Diet caffeine-free Coke |
10 Triscuit crackers with cheese slices
|
|
|
What causes T1DM?
|
Auto-immune disease caused by Th1 cell recruitment of CD8 T cells and destruction of beta-cells with cytokines
|
|
|
What are the symptoms of T1DM?
|
- Increased thirst
- Urination - Hunger - Weight loss - Fatigue |
|
|
What are the signs of Diabetic Ketoacidosis (DKA)?
|
- Severe hyperglycemia
- Ketoacidemia - Dehydration leading to hypovolemic shock |
|
|
What is the most common regimen of insulin?
|
Multiple daily injections (MDI):
- Long acting insulin, such as Glargine or Detemir AND - Short acting insulin w/ meals, such as Lispro or Aspart |
|
|
What is the result of activation of destructive auto-immunity in T1DM?
|

- Progressive loss of β cell mass
- With time, the β cell mass is so low that insulin deficiency results in DM |
|
|
What is the period of time after DM develops but some insulin secretion is still present?
|

Honey-moon phase - period of time after the presentation of T1DM when the glucose control is relatively easy to achieve
|
|
|
What happens after all β cell mass is lost in T1DM?
|

- Glucose levels are more difficult to control, resulting in increased glucose variability
- Glucagon regulation is also abnormal, as the paracrine function of insulin has been lost - Sometimes the inflammatory destruction involves the entire islet, but usually there are residual α- and δ-cells |
|
|
How does the rate of β-cell decline compare in adults vs children?
|
Rate of β-cell decline is slower in adults, relative to children
|
|
|
What is the goal of therapy for patients w/ T1DM?
|
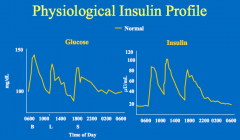
Physiological insulin replacement (insulin increases after glucose increases, and declines as glucose declines)
|
|
|
In a normal person, what is the relationship of insulin to eating?
|
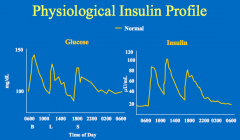
- With eating, there is an increase in insulin levels
- Insulin sends an anabolic signal to the tissues - The insulin then returns to baseline (fasting levels) which is not zero |
|
|
What are the categories of insulin replacement? Relationship?
|
- Basal
- Base - Correction Factor Bolus = Base + Correction |
|
|
What is the term for the amount of insulin needed when not active and not eating?
|
Basal insulin replacement
|
|
|
What is the term for the amount of insulin needed to cover the calories of a meal
|
Base insulin replacement
|
|
|
What is the term for the amount of insulin added or subtracted to account for deviation from the desired range?
|
Correction factor
Bolus = Base + Correction |
|
|
What is the other term for physiological insulin replacement?
|
Basal-Bolus insulin replacement
|
|
|
What are the characteristics of Regular Insulin? What happens to it when injected?
|
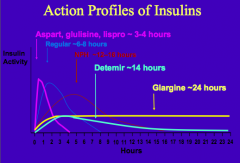
- Human insulin made w/ recombinant DNA technology
- Human insulin self-aggregates as a hexamer - Upon injection into subcutaneous tissue, the hexamers must dissociate to monomers prior to absorption - Lasts ~6-8 hrs |
|
|
What are the human insulin analogs that are more rapidly absorbed?
|
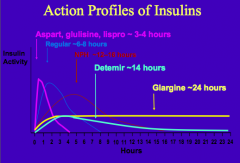
- Aspart
- Glulisine - Lispro - Lasts ~3-4 hrs |
|
|
What are the characteristics of Aspart, Glulisine, and Lispro insulin? What happens to it when injected?
|
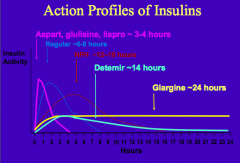
- Human insulin analogs
- Their amino acid sequence is modified to prevent self-aggregation - They exist as monomers that are more rapidly absorbed - Lasts ~3-4 hrs |
|
|
What is the human insulin analog with zinc and protamine? Why are these added?
|
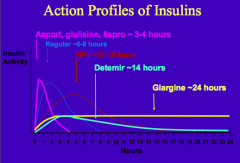
NPH = Neutral Protamine Hagedorn
- Zinc and Protamine are added to increase aggregation - Lasts ~12-16 hrs |
|
|
What is the human insulin analog with a fatty acid side-chain added? Why is this added?
|
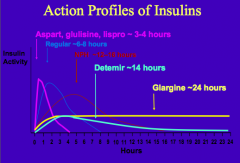
Detemir Insulin
- Promotes binding to albumin, which prolongs its action profile - Lasts ~14 hrs |
|
|
What is the longest lasting insulin analog? Characteristics upon injection?
|
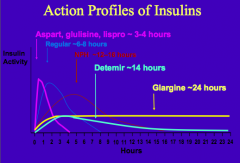
Glargine Insulin:
- In the syringe at pH 4 it is soluble - Upon injection into the subcutaneous tissue w/ pH 7.4, glargine aggregates and is slowly absorbed - Lasts ~24 hrs |
|
|
What are the types of insulin from shortest acting to longest acting
|
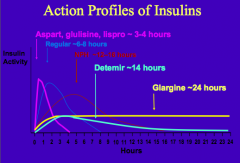
- 3-4 hrs: Aspart, Glulisine, Lispro
- 6-8 hrs: Regular Insulin - 12-16 hrs: NPH - 14 hrs: Detemir - 24 hrs: Glargine |
|
|
Where is insulin injected?
|
Abdomen - more uniformly absorbed
|
|
|
What is a potential complication of insulin therapy in the abdomen? How can this be prevented?
|
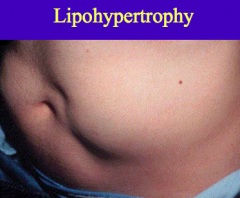
Lipohypertrophy:
- Occurs when insulin is injected repeatedly in the same small area - It is a direct consequence of insulin's lipogenic action on the subcutaneous adipose tissue *Can be avoided w/ site rotation |
|
|
What are two typical insulin regimens used to treat DM?
|
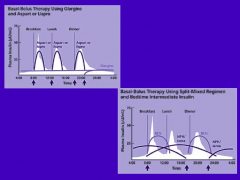
1. Glargine provides basal insulin w/ Aspart or Lispro providing the bolus insulin to cover the calories of the meal (Glargine can not be mixed w/ other insulin preps b/c it requires a low pH prior to injection)
2. Regular insulin and NPH are used to give a basal bolus pattern; NPH in morning covers basal needs and the calories at lunch; NPH given at bedtime to cover the night-time basal requirements |
|
|
When does diabetic ketoacidosis occur?
|
In the presence of absolute insulin deficiency
- T1DM - T2DM (in setting of poor calorie consumption, prolonged poor glycemic control, and often concomitant illness) |
|
|
How can patients w/ T1DM prevent diabetic ketoacidosis?
|
Never miss their insulin dose
|
|
|
When can a T2DM patient get Diabetic Ketoacidosis?
|
Absolute Insulin Deficiency d/t:
- Poor calorie consumption - Prolonged poor glycemic control (reduces the ability of the pancreas to secrete insulin) - Often concomitant illness |
|
|
What is the principle of glucose toxicity?
|
Prolonged poor glycemic control further reduces the ability of the pancreas to secrete insulin
|
|
|
How do patients compensate for Diabetic Ketoacidosis? Function?
|
- Compensatory hyperpnea (increased tidal volume and breathing rate) to try to blow off more CO2 to fix the metabolic acidosis
- Hyperpnea is in the pattern of Kussmaul respirations, which is a deep, rhythmic breathing |
|
|
If your diabetic patient is having Kussmaul respirations (deep, rhythmic breathing) what do you think?
|
They may have diabetic ketoacidosis - the compensatory hyperpnea is to try to blow off the extra CO2 to fix the metabolic acidosis
|
|
|
How do ketone bodies get formed?
|
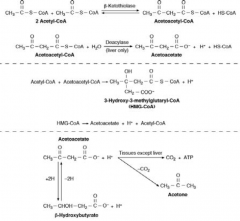
- Breakdown of FFA through β-oxidation
- Leads to accumulation of acetyl-CoA - In the liver, acetyl-CoA is converted to ketone bodies: acetoacetate, β-hydroxybutyrate and acetone |
|
|
What are the ketone bodies?
|
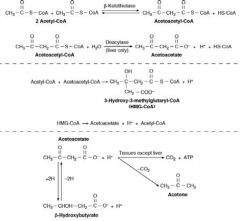
- Acetoacetate
- β-hydroxybutyrate - Acetone |

