![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
122 Cards in this Set
- Front
- Back
|
Where are the adult adrenal glands located?
|
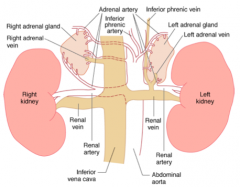
- Retroperitoneum
- Above or medial to the upper poles of the kidneys |
|
|
What are the zones of the adrenal glands?
|
Cortex (90% of mass)
- Glomerulosa - Fasciculata - Reticularis Medulla (10% of mass) |
|
|
What is the blood supply to the adrenal glands? Significance?
|
Main arterial supply from branches of:
- Inferior phrenic artery - Renal arteries - Aorta *Prevents ischemic injury to adrenal gland during under perfusion |
|
|
What is the blood drainage to the adrenal glands? Significance?
|
- R adrenal vein drains directly into the posterior aspect of the vena cava
- L adrenal vein drains into the L renal vein before entering the IVC * Each vein is vulnerable to venous thrombosis and non-traumatic hemorrhage of adrenal glands is often d/t bilateral or unilateral venous thrombosis creating a vascular dam effect |
|
|
What controls steroid production in the zona fasciculata and reticularis?
|
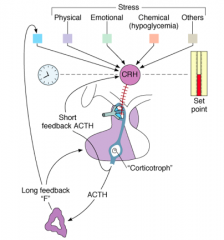
- CRH from the hypothalamus stimulates ACTH in a pulsatile manner
- ACTH stimulates the adrenal steroids |
|
|
What disease process has unregulated cortisol secretion?
|
*Cushing's Disease - an ACTH-secreting pituitary tumor
- Manifested by deregulated cortisol secretion since the set point for negative feedback on ACTH is increased *Stressors may also stimulate ACTH secretion overriding glucocorticoid negative feedback |
|
|
When is there peak cortisol secretion?
|
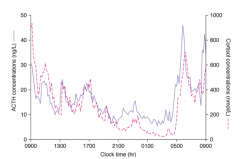
Diurnal rhythm which causes a peak before awakening and a decline as the day progresses
|
|
|
What is ACTH synthesized from?
|
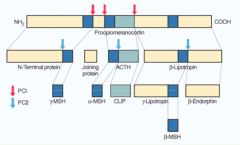
- Precursor molecule: Pro-opiomelanocortin (POMC) within the ACTH-secreting cells in the pituitary gland corticotroph
- Convertase enzymes direct synthesis and processing of POMC - ACTH is one of the segments of POMC |
|
|
What causes increased skin pigmentation in primary adrenal insufficiency?
|
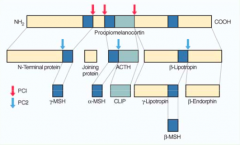
Elevated ACTH; ACTH contains the amino acid sequence of alpha-melanocyte stimulating hormone (αMSH) is within the peptide hormone complex
|
|
|
What does the cortisol synthetic pathway require?
|
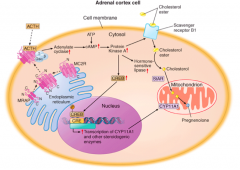
- ACTH-stimulated cholesterol import into the mitochondrion
- Action is initiated by Steroidogenic Acute Regulatory (StAR) protein, which shuttles cholesterol from outer to inner mitochondrial membrane |
|
|
What is the action of the Steroidogenic Acute Regulatory (StAR) protein)?
|
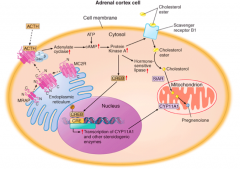
Initiates the ACTH-stimulated cholesterol import from the outer to the inner mitochondrial membrane
|
|
|
What stimulates the aldosterone synthesis pathway in the zona glomerulosa?
|
- Angiotensin II
- Potassium - Acutely, by ACTH |
|
|
What kind of enzymes are the steroidogenic enzymes? Location?
|
Cytochrome P450 enzymes
- Located in the mitochondrion or in the ER membrane |
|
|
Which steroidogenic enzymes are located in the mitochondrion?
|
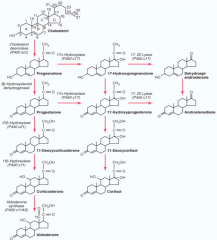
- Side chain cleavage enzyme
- 11-hydroxylase - Aldosterone synthase |
|
|
Which steroidogenic enzymes are located in the ER membrane?
|
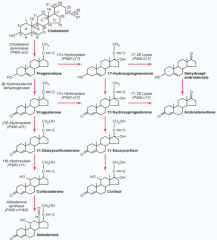
- 17-hydroxylase
- 21-hydroxylase |
|
|
What is the first precursor derived from cholesterol for steroid synthesis? What enzyme makes this conversion?
|
Pregnenolone
- Generated by Cholesterol Side Chain Cleavage Enzyme (a CYP enzyme) |
|
|
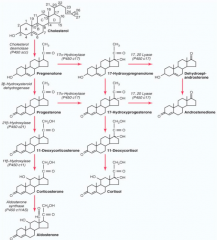
How is Cholesterol converted to Glucocorticoids?
|
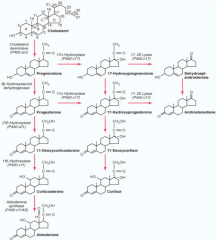
1. Cholesterol → Pregnenolone by Side Chain Cleavage Enzyme
2. Pregnenolone → Progesterone by 3β-Hydroxysteroid Dehydrogenase (3-βHSD2) 3. Progesterone → 17-Hydroxyprogesterone by 17-Hydroxylase 4. 17-Hydroxyprogesterone → 11-Deoxycortisol by 21-Hydroxylase 5. 11-Deoxycortisol → Cortisol by 11-Hydroxylase |
|
|
What are the enzymes on the progression from Cholesterol to Cortisol?
|
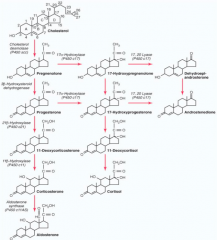
1. Side Chain Cleavage Enzyme
2. 3β-Hydroxysteroid Dehydrogenase (3-βHSD2) 3. 17-Hydroxylase (P450c17) 4. 21-Hydroxylase (P450c21) 5. 11-Hydroxylase (P450c11) |
|
|
What are the molecules on the progression from Cholesterol to Cortisol?
|
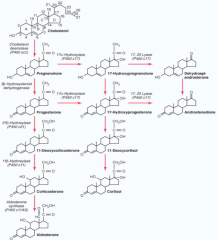
Cholesterol →
1. Pregnenolone 2. Progesterone 3. 17-Hydroxyprogesterone 4. 11-Deoxycortisol 5. Cortisol |
|
|
How is Cholesterol converted to Mineralocorticoids?
|
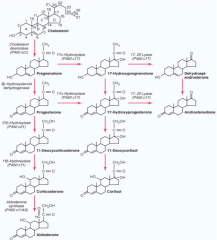
1. Cholesterol → Pregnenolone by Side Chain Cleavage Enzyme
2. Pregnenolone → Progesterone by 3β-Hydroxysteroid Dehydrogenase (3-βHSD2) 3. Progesterone → 11-Deoxycorticosterone by 21-Hydroxylase 4. 11-Deoxycorticosterone → Corticosterone by 11-Hydroxylase 5. Corticosterone → Aldosterone by Aldosterone Synthase |
|
|
What are the enzymes on the progression from Cholesterol to Aldosterone?
|
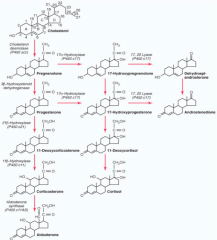
1. Side Chain Cleavage Enzyme
2. 3β-Hydroxysteroid Dehydrogenase (3-βHSD2) 3. 21-Hydroxylase (P450c21) 4. 11-Hydroxylase (P450c11) 5. Aldosterone Synthase (P450c11AS) |
|
|
What are the molecules on the progression from Cholesterol to Aldosterone?
|
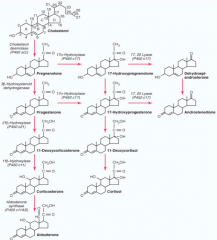
1. Pregnenolone
2. Progesterone 3. 11-Deoxycorticosterone 4. Corticosterone 5. Aldosterone |
|
|
What happens to cortisol in the circulation?
|
Binds to plasma proteins
- Mainly to Corticosteroid-Binding Globulin (CBG) - A bit to Albumin |
|
|
How much of cortisol is free in the circulation? Implications?
|
Only 5% is circulating free; bound steroids are biologically inactive
|
|
|
How do you measure the free cortisol? Utility?
|
- Can be done in saliva and is independent of the salivary flow rates
- Widely used for the assessment of the hypothalamic-pituitary-adrenal axis, particularly in states of suspected hypercortisolism |
|
|
How is cortisol metabolized and excreted?
|
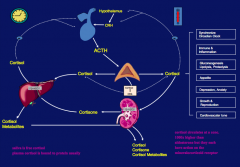
- Cortisol is metabolized in the liver and kidney
- Some metabolites are excreted in the urine |
|
|
What is the first step in the metabolism of cortisol? Where does it take place?
|
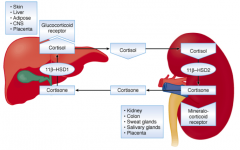
- Cortisol is metabolized to Cortisone
- In kidney by 11β-Hydroxysteroid Dehydrogenase type 2 (11β-HSD2) - Cortisol can bind mineralocorticoid receptor, but Cortisone can't |
|
|
What is the function of conversion of Cortisol to Cortisone in the liver?
|
- Protects the mineralocorticoid receptor on the tubules of the distal nephron from the action of cortisol
- Cortisol has the same affinity for the mineralocorticoid receptor as aldosterone, but cortisol circulates at 1000x the total concentration |
|
|
How does cortisol affect the mineralocorticoid receptor?
|
Cortisol has the same affinity for the mineralocorticoid receptor as aldosterone, but cortisol circulates at 1000x the total concentration
|
|
|
What clinical circumstances may alter the protective effect of 11β-HSD2 on cortisol?
|
- Large increases in cortisol secretion may overwhelm the intra-renal metabolism of cortisol
- This would cause significant mineralocorticoid effects such as HTN and hypokalemia - Seen in Cushing Syndrome |
|
|
What is important about synthetic glucocorticoids (eg, dexamethasone)?
|
Does not have a high affinity for the mineralocorticoid receptor
|
|
|
What happens to the Cortisone produced in the kidney that is not excreted in the urine?
|
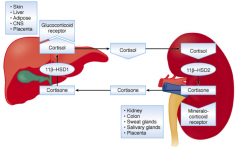
It can be converted back into cortisol by 11β-hydroxysteroid dehydrogenase type 1 in the liver and visceral fat
|
|
|
Where are 11β-hydroxysteroid dehydrogenase type 1 and type 2 found? Effect?
|
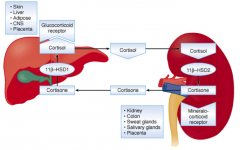
- Type 1: in liver converts cortisone to cortisol
- Type 2: in kidney converts cortisol to cortisone |
|
|
What does cortisol bind to and where to exert its effect?
|
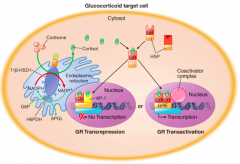
Cortisol interacts with the intracellular Glucocorticoid Receptor (GR)
|
|
|
What is the mechanism of action for cortisol?
|
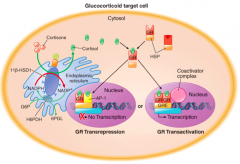
- Cortisol binds in the cytosol of target cells to GR which results in dissociation of heat shock proteins (HSP)
- Cortisol bound GR dimerizes and translocates to the nucleus to activate glucocorticoid response elements (GRE) - Enhances transcription of genes |
|
|
How does cortisol mediate an anti-inflammatory effect?
|
- Cortisol-bound GR can form heterodimers with transcription factors such as AP-1 or NF-B
- This transrepresses pro-inflammatory genes\ |
|
|
Which hormone besides cortisol can bind to the glucocorticoid receptors? Effect?
|
- Corticosterone (precursor of aldosterone)
- It has much weaker binding activity |
|
|
How can patients with a 17-hydroxylase deficiency compensate for their lack of cortisol?
|
They have higher concentrations of Corticosterone (precursor of Aldosterone) which has weak activity on the Glucocorticoid Receptor
|
|
|
What is the specificity of the steroid receptors? Implications?
|
- Steroid receptors may not always be specific and may occasionally bind other steroids causing unexpected clinical effects?
- Eg, high dose potent progesterone agents may occupy glucocorticoid receptor and cause cortisol-like effects |
|
|
What steroid compound can stimulate appetite in cancer patients? How?
|
Progesterone compound, Megestrol Acetate - stimulates appetite and causes suppression of ACTH and cortisol
|
|
|
What drug can be used for the treatment of endogenous hypercortisolism?
|
Mifepristone - a glucocorticoid receptor antagonist
|
|
|
What are the targets of glucocorticoids that impacts intermediary metabolism?
|
- Liver
- Adipose tissue - Skeletal muscle - Plasma glucose |
|
|
How do glucocorticoids affect the metabolism in the liver? What are the effects of hypercortisolism and hypocortisolism?
|
- Increases expression of gluconeogenic enzymes
- Hypercortisolism: increases hepatic glucose output, together w/ insulin, increases hepatic glycogen stores - Hypocortisolism: decreases hepatic glucose output and glycogen stores |
|
|
How do glucocorticoids affect the metabolism in the adipose tissue? What are the effects of hypercortisolism and hypocortisolism?
|
- Permissive for lipolytic signals leading to elevated plasma FFA to fuel gluconeogenesis
- Hypercortisolism: central / truncal obesity, moon facies, and buffalo hump - Hypocortisolism: decreases adiposity and decreases lipolysis |
|
|
How do glucocorticoids affect the metabolism in skeletal muscle? What are the effects of hypercortisolism and hypocortisolism?
|
- Degrades fibrillar muscle proteins by activating ubiquitin pathway, providing amino acids for gluconeogenesis
- Hyper-cortisolism: muscle weakness and wasting, mainly in proximal muscles; increased urinary nitrogen excretion (urea from amino acids) - Hypo-cortisolism: muscle weakness, decreased muscle glycogen stores; decreased urinary nitrogen excretion |
|
|
How do glucocorticoids affect the metabolism in the plasma glucose? What are the effects of hypercortisolism and hypocortisolism?
|
- Maintains plasma glucose during fasting (anti-hypoglycemic action); increases plasma glucose during stress
- Hyper-cortisolism: impairs glucose tolerance, insulin-resistant diabetes mellitus, increases plasma glucose d/t peripheral glucose utilization and increases hepatic glucose output - Hypo-cortisolism: hypoglycemia, increases insulin sensitivity |
|
|
What are the targets of glucocorticoids that impact calcium homeostasis?
|
- Kidney
- Bone, cartilage - GI tract |
|
|
How do glucocorticoids affect calcium homeostasis in the kidney? What are the effects of hypercortisolism and hypocortisolism?
|
- Decreases reabsorption of calcium
- Hyper-cortisolism: hypercalciuria without hypercalcemia leading to secondary hyper-PTH - Hypo-cortisolism: retardation of bone growth mainly through decreased GH, hypercalcemia possible |
|
|
How do glucocorticoids affect calcium homeostasis in the bone/cartilage? What are the effects of hypercortisolism?
|
- Inhibits collagen synthesis and bone deposition
- Hyper-cortisolism: retards bone growth and bone age by direct action and by decreasing GH; causes osteoporosis in adults |
|
|
How do glucocorticoids affect calcium homeostasis in the GI tract?
|
Inhibits calcium, magnesium, and phosphate absorption
|
|
|
What are the endocrine targets of glucocorticoids?
|
- Hypothalamus and Pituitary
- Pancreas - Adrenal Medulla |
|
|
How do glucocorticoids affect the hypothalamus and pituitary? What are the effects of hypercortisolism and hypocortisolism?
|
- Decreases endogenous opioid production and depresses gonadotroph responsiveness to GnRH, stimulates GH expression by pituitary, inhibits GH secretion by hypothalamus
- Hyper-cortisolism: scanty menses d/t suppressed gonadotroph sensitivity go GnRH, also suppresses GH secretion by hypothalamic action, minimal suppression of TRH-TSH axis - Hypo-cortisolism: scanty menses by upregulated CRH-endogenous opioid pathway-mediated suppression of GnRH, suppressed GH secretion, hypothyroidism (if present) is d/t autoimmune mechanism |
|
|
How do glucocorticoids affect the pancreas? What are the effects of hypercortisolism and hypocortisolism?
|
- Inhibits insulin secretion by decreasing efficacy of cytoplasmic Ca2+ on exocytotic process
- Hyper-cortisolism: absolute hyperinsulinemia with relative hypoinsulinemia (lower plasma insulin than expected for the degree of hyperglycemia) - Hypo-cortisolism: absolute hypoinsulinemia with relative hyperinsulinemia |
|
|
How do glucocorticoids affect the adrenal medulla? What are the effects of hypercortisolism and hypocortisolism?
|
- Increases PNMT expression and activity (epinephrine synthesis)
- Hyper-cortisolism: increases response to sympathoadrenal activation - Hypo-cortisolism: decreases response to sympathoadrenal activation |
|
|
What are the immune system targets of glucocorticoids?
|
- Thymus / lymphocytes
- Granulocytes - Erythrocytes |
|
|
How do glucocorticoids affect the thymus / lymphocytes? What are the effects of hypercortisolism and hypocortisolism?
|
- Involution of the thymus
- Hyper-cortisolism: immunocompromised state, lymphocytopenia - Hypo-cortisolism: relative lymphocytosis |
|
|
How do glucocorticoids affect granulocytes? What are the effects of hypercortisolism and hypocortisolism?
|
- Demargination of neutrophils by suppressing the expression of adhesion molecules
- Hyper-cortisolism: granulocytosis and eosinopenia for peripheral blood - Hypo-cortisolism: granulocytopenia and eosinophilia for peripheral blood |
|
|
How do glucocorticoids affect erythrocytes? What are the effects of hypercortisolism?
|
- No significant effect
- Hyper-cortisolism: increased hemoglobin and hematocrit |
|
|
How do glucocorticoids affect skin and connective tissue? What are the effects of hypercortisolism and hypocortisolism?
|
- Anti-proliferative for fibroblasts and keratinocytes
- Hyper-cortisolism: easy bruisability d/t dermal atrophy, striae or sites of increased tension, especially sites of adipose tissue accumulation; poor wound healing; hirsutism and acne are d/t ACTH-mediated increase of adrenal androgens; hyper-pigmentation is direct effect of ACTH on melanocortin 1 receptors - Hypo-cortisolism: darkening of the skin is d/t ACTH-mediated stimulation of melanocortin 1 receptors; vitiligo may occur d/t direct auto-immune destruction of melanocytes in circumscribe areas |
|
|
How do glucocorticoids affect the heart? What are the effects of hypercortisolism and hypocortisolism?
|
- Increased contractility
- Hyper-cortisolism: HTN - Hypo-cortisolism: lower peripheral resistance, HTN w/ further postural decrease in blood pressure (orthostatic hypotension); low-voltage ECG |
|
|
How do glucocorticoids affect the vasculature?
|
Increased vascular reactivity to vasoconstrictors (catecholamines and AngII)
|
|
|
How do glucocorticoids affect Na+, K+, and the ECF volume? What are the effects of hypercortisolism and hypocortisolism?
|
- Increase GFR and non-physiologic actions on mineralocorticoid receptors
- Hyper-cortisolism: hypokalemic alkalosis, increased ECF volume d/t MR activity - Hypo-cortisolism: hyponatremia, hyperkalemic acidosis, and decreased ECF volume, mainly d/t loss of MR activity |
|
|
What psychiatric parameters of CNS function are affected by glucocorticoids?
|
- Mood
- Appetite - Sleep - Memory - Eye |
|
|
How do glucocorticoids affect the mood? What are the effects of hypercortisolism and hypocortisolism?
|
- Eucortisolemia maintains emotional balance
- Hyper-cortisolism: initially euphoria, but later long-term depression and psychosis - Hypo-cortisolism: depression |
|
|
How do glucocorticoids affect the appetite? What are the effects of hypercortisolism and hypocortisolism?
|
- Increases appetite
- Hyper-cortisolism: hyperphagia - Hypo-cortisolism: decreased appetite in spite of improved taste and smell |
|
|
How do glucocorticoids affect the sleep? What are the effects of hypercortisolism?
|
- Suppression of REM sleep
- Hyper-cortisolism: sleep disturbances |
|
|
How do glucocorticoids affect the memory? What are the effects of hypercortisolism?
|
- Sensitizes hippocampal glutamate receptors, induces atrophy of dendrites
- Hyper-cortisolism: impaired memory, bilateral hippocampal atrophy |
|
|
How do glucocorticoids affect the eye? What are the effects of hypercortisolism and hypocortisolism?
|
- Increases intraocular pressure
- Hyper-cortisolism: cataracts and increased intraocular pressure - Hypo-cortisolism: decreased intraocular pressure |
|
|
What is adrenal insufficiency? What can cause it?
|
- Inadequate production of cortisol from the adrenal cortex
Causes: - Primary - disease process in adrenal gland - Secondary - disease process in pituitary gland - Tertiary - disease process in hypothalamus |
|
|
What are the signs of untreated secondary or tertiary adrenal insufficiency?
|
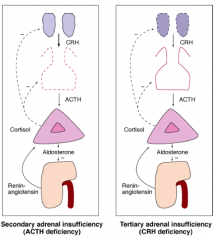
- Low cortisol
- Low or inappropriately normal ACTH levels (remember, ACTH should be high) |
|
|
What are the signs of untreated primary adrenal insufficiency?
|
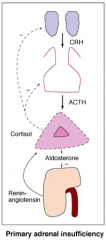
- Low cortisol
- Elevated ACTH - Also, missing aldosterone (hypotension is more common and hyperkalemia may be present) - Elevated Renin |
|
|
What are the signs and symptoms of adrenal insufficiency?
|
- Fatigue, malaise, lack of energy
- GI: nausea, vomiting, anorexia → weight loss - Hypotension → dizziness, orthostasis |
|
|
What are the signs and symptoms specific to PRIMARY adrenal insufficiency?
|
- Increased skin pigmentation
- Salt craving |
|
|
What are the lab abnormalities in adrenal insufficiency?
|
* Hyponatremia
- Hyperkalemia (primary) - Hypercalcemia, hypoglycemia (rare in adults) - Lymphocytosis |
|
|
What are the potential imaging abnormalities in adrenal insufficiency?
|
- Bilateral adrenal enlargement
- Pituitary mass |
|
|
What concurrent medical problems are associated with adrenal insufficiency?
|
- Severe critical illness with hypotension
- Pituitary disease - Traumatic brain injury - Brain radiation |
|
|
What drugs are associated with adrenal insufficiency?
|
- Withdrawal from corticosteroids (oral, inhaled, topical, parenteral)
- Narcotics (suppress CRH/ACTH - common cause of tertiary adrenal insufficiency) - Adrenostatic / lytic: ketoconazole, etomidate, mitotane - Glucocorticoid receptor antagonist: mifepristone |
|
|
What should you think if you see a patient with hyponatremia?
|
Adrenal Insufficiency (both primary and secondary) - better check cortisol level
|
|
|
What is a common cause of tertiary adrenal insufficiency?
|
Narcotics - suppress CRH/ACTH
|
|
|
What genetic abnormalities are associated with adrenal insufficiency?
|
- Congenital Adrenal Hyperplasia
- Adrenoleukodystrophy (X-linked disorder w/ accumulation of very long chain fatty acids in adrenal glands and the brain) |
|
|
Your patient presents with weight loss, fatigue, postural hypotension, hyperpigmentation, and hyponatremia, what do you suspect?
|
Adrenal Insufficiency
|
|
|
You suspect your patient has adrenal insufficiency based on presentation (weight loss, fatigue, postural hypotension, hyperpigmentation, and hyponatremia), what do you test first?
|
- Plasma cortisol 30-60 minutes after a 250 µg cosyntropin IM or IV (cortisol < 500 nmol/L)
- CBC, serum sodium, potassium, creatinine, urea, TSH |
|
|
What plasma cortisol result would indicate adrenal insufficiency 30-60 min after 250 µg cosyntropin IM or IV?
|
< 500 nmol/L
|
|
|
Once you've established your patient has adrenal insufficiency with a plasma cortisol 30-60 min after 250 µg cosyntropin test, how do you determine the cause?
|
- Plasma ACTH
- Plasma Renin - Serum Aldosterone |
|
|
What would indicate a diagnosis of Primary Adrenal Insufficiency?
|
- High ACTH
- High PRA (plasma renin activity) - Low Aldosterone |
|
|
How do you treat Primary Adrenal Insufficiency (high ACTH, high plasma renin activity, and low aldosterone)?
|
Glucocorticoid and Mineralocorticoid replacement
|
|
|
What should you first evaluate as a possible cause of primary adrenal insufficiency?
|
Adrenal Auto-Antibodies
- If positive, auto-immune adrenalitis or auto-immune polyglandular syndrome (APS) |
|
|
What should you evaluate if your patient with primary adrenal insufficiency is negative for adrenal auto-antibodies?
|
- Chest x-ray
- Serum 17OHP - In men: plasma very long chain fatty acids (VLCFA) - Adrenal CT |
|
|
What does it mean if your patient with primary adrenal insufficiency has a positive chest x-ray?
|
- Adrenal infection (Tuberculosis)
- Infiltration (eg, Lymphoma) OR - Hemorrhage |
|
|
What does it mean if your patient with primary adrenal insufficiency has an elevated 17OHP?
|
Congenital Adrenal Hyperplasia (17OHP ↑)
|
|
|
What is likely the cause of adrenal insufficiency if they have a negative chest x-ray and normal serum 17OHP?
|
- Auto-immune adrenalitis most likely diagnosis
- In men, consider adrenoleukodystrophy (VLCFA ↑) |
|
|
What would indicate a diagnosis of Secondary Adrenal Insufficiency?
|
- Low or normal ACTH
- Normal Plasma Renin Activity - Normal Aldosterone |
|
|
How do you treat Secondary Adrenal Insufficiency?
|
Glucocorticoid replacement
|
|
|
What should you first evaluate to determine a possible cause of secondary adrenal insufficiency?
|
MRI of Pituitary:
- Positive: Hypothalamic-Pituitary mass lesion |
|
|
If a patient has secondary adrenal insufficiency (low/normal ACTH, normal plasma renin activity, and normal aldosterone), and their MRI of the pituitary is negative, what should you consider?
|
- History of exogenous glucocorticoid treatment
- History of head trauma - Consider isolated ACTH deficiency |
|
|
How should you first evaluate a suspected adrenal insufficiency? When? Why?
|
Measure a morning level of cortisol (this is when it should be at its peak)
- Range of normal: 6 - 18 µg/dL - Adrenal Insufficiency: < 5 µg/dL - >14 µg/dL excludes adrenal insufficiency - 5-14 µg/dL - need to have a stimulatory test of adrenal reserve w/ synthetic ACTH (Cosyntropin) followed by a cortisol sampling at 30 and/or 60 minutes (at any time of day) |
|
|
What constitutes a normal cortisol response to ACTH (Cosyntropin)?
|
Peak response at 30-60 minutes > 18 µg/dL (500 nm/L)
|
|
|
What constitutes an abnormal cortisol response to ACTH (Cosyntropin)? What should you do next?
|
- Peak cortisol response to ACTH / Cosyntropin is < 18 µg/dL or < 500 nmol/L
AND - Basal morning cortisol is < 5 µg/dL, then you should: * Measure plasma ACTH to distinguish primary from secondary adrenal insufficiency |
|
|
If your patient has a low cortisol response to ACTH and has a basal morning cortisol < 5 µg/dL, then what should you do next? Implications?
|
Measure Plasma ACTH:
- Elevated: primary adrenal insufficiency - Low or Normal: secondary adrenal insufficiency |
|
|
When assessing someone for adrenal insufficiency in a critically ill or chronically ill patient, what do you need to consider during evaluation of their labs?
|
- These patients may have low binding problems (ie, CBG)
- Total cortisol concentration may be low and the "free" or biologically active cortisol may be normal - Assessment of "free" cortisol can be done in saliva, plasma, or by formula concentration med if conc. of CBG is known |
|
|
When assessing someone for adrenal insufficiency who is taking estrogen / oral contraceptives, what do you need to consider during evaluation of their labs?
|
- These patients will have a significant increase in total cortisol levels that reflect an increase in CBG rather than any alteration of adrenal function
- Therefore even if they have a "normal" cortisol, this may be "low" for them given that the estrogen should be making their cortisol even higher |
|
|
What is a sensitive marker of adrenal reserve?
|
Adrenal Androgen Production (DHEAS) - a normal level is very unusual in patients with any type of adrenal insufficiency
|
|
|
What is the most common cause of Primary Adrenal Insufficiency?
|
Auto-immune Polyglandular Syndromes
|
|
|
Besides auto-immune polyglandular syndrome, what are some other causes of Primary Adrenal Insufficiency?
|
- Metastases (from lung, breast, melanoma, GI)
- Primary adrenal lymphoma - Bilateral adrenal hemorrhages (associated w/ coagulopathies such as anti-coagulation therapy, anti-phospholipid syndrome, heparin-induced thrombocytopenia) and usually d/t bilateral adrenal vein thrombosis - Infection: TB, fungi (histoplasmosis, coccidiomycosis), HIV - Genetic: congenital adrenal hyperplasia, familial glucocorticoid deficiency, adrenoleukodystrophy - Infiltrative disorders: amyloidosis, hemochromatosis - Drugs: Ketoconazole, Metyrapone, Mitotane, Etomidate |
|
|
What malignancies can cause Primary Adrenal Insufficiency?
|
- Metastatic spread from lung, breast, melanoma, or GI
- Primary adrenal lymphoma - Fairly unusual because it is rarely bilateral |
|
|
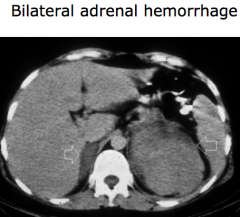
What can cause adrenal hemorrhage bilaterally? What would this cause?
|
- Primary Adrenal Insufficiency
- Associated with coagulopathies (anti-coagulation therapy, anti-phospholipid syndrome, heparin-induced thrombocytopenia) and usually d/t bilateral adrenal vein thrombosis |
|
|
What infections can cause Primary Adrenal Insufficiency?
|
- Tuberculosis
- Fungi (Histoplasmosis and Coccidiomycosis) - HIV |
|
|
What genetic abnormalities can cause Primary Adrenal Insufficiency?
|
- Congenital Adrenal Hyperplasia
- Familial Glucocorticoid Deficiency - Adrenoleukodystrophy |
|
|
What infiltrative disorders can cause Primary Adrenal Insufficiency?
|
- Amyloidosis
- Hemochromatosis |
|
|
What drugs can cause Primary Adrenal Insufficiency?
|
- Ketoconazole
- Metyrapone - Mitotane - Etomidate |
|
|
What are the causes of secondary adrenal insufficiency?
|
- Withdrawal from exogenous corticosteroid therapy
- Pituitary / hypothalamic disease |
|
|
What pituitary / hypothalamic disease can cause Secondary Adrenal Insufficiency?
|
Hypophysitis may cause isolated ACTH deficiency
- Auto-immune - Granulomatosis - Drug-induced (Ipilimumab) |
|
|
How do you treat Primary Adrenal Insufficiency?
|
- Hydrocortisone: morning (bigger dose) and afternoon (smaller dose); should have injectable version available for emergency use
- Fludrocortisone: given daily (given for mineralocorticoid replacement) |
|
|
What do you need to monitor in a patient taking Hydrocortisone?
|
- Monitor sense of well-being
- Plasma ACTH should remain elevated even w/ adequate hydrocortisone - Low/normal ACTH levels may reflect over-replacement |
|
|
What do you need to monitor in a patient taking Fludrocortisone?
|
- Monitor electrolyte composition
- Monitor plasma renin activity (<5 ng/mL/min) |
|
|
What patient education should you do for a patient with Primary Adrenal Insufficiency?
|
- Identification card / medical alert bracelet
- Sick day management: 3x3 → triple the dose for three days or until illness resolves |
|
|
How should you treat a patient with Primary Adrenal Insufficiency in an acute adrenal crisis?
|
- Administer hydrocortisone 100 mg IV every 6 hours for 24 hours
- When stable decrease to 50 mg every 6 hours and then taper to maintenance as clinically warranted - Support w/ isotonic, glucose containing IV fluids to replace volume |
|
|
How should you treat a patient with Primary Adrenal Insufficiency undergoing surgery?
|
- Correct electrolytes, BP, and hydration status if necessary
- Give hydrocortisone 100 mg IM or IV on call to OR - Hydrocortisone 50 mg every 6-8 hours and then taper judiciously to maintenance |
|
|
How do you treat a patient with Secondary Adrenal Insufficiency?
|
- Hydrocortisone: lower doses needed than in primary AI
|
|
|
Do patients with primary or secondary adrenal insufficiency need less hydrocortisone therapy? Why?
|
- Secondary Adrenal Insufficiency requires lower doses
- These patients usually still have some cortisol secretion from adrenals |
|
|
Does Primary or Secondary Adrenal insufficiency require Fludrocortisone? Why?
|
- Primary Adrenal Insufficiency requires Fludrocortisone
- This has mineralocorticod activity - Secondary AI does not need MR replacement since renin-angiotensin-aldosterone system and zona glomerulosa remain intact |
|
|
How do you treat a patient with Primary vs Secondary Adrenal Insufficiency for sick days, adrenal crises, and surgical steroid coverage?
|
Same therapies
|

