![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
57 Cards in this Set
- Front
- Back
|
What are the most common elements that make up a cell? |
Hydrogen, Carbon, Nitrogen, and Oxygen make up 99% of the cell. Na, Mg, P, S, Cl, K, and Ca make up 0.9%. |
|
|
Life uses elements that are ______, ________, and ________. |
Common, reactive, and form strong bonds. |
|
|
Which of the elements most common in living organisms makes especially stable bonds? Known as the "backbone of life." |
Carbon |
|
|
What are macromolecules? |
Large molecules containing a large number of atoms. Key structural and functional components of cells. |
|
|
What are the four main classes of macromolecules? |
Polysaccharides: Sugar is the subunit, and they have 2 structural types (Branched and linear) Proteins: Amino acids are the subunit Nucleic Acids: Nucleotides are the subunits. DNA and RNA Lipids: many structural types. Not a polymer like the other 3 |
|
|
What is a polymer? |
A chain of the same or similar molecules. Ex: Proteins |
|
|
What are the two classes of peptides, and how are they categorized? |
Oligopeptides: 2-20 aa. Ex: di-, tri-, and tetrapeptide, cell-to-cell signals, neuropeptides (opiate peptides, substance P, vasopressin) Polypeptides/Proteins: 30+ aa. Typically several hundred aa. Categorized by size |
|
|
What 3 traits do proteins share? |
1. All adopt at least 2 stable conformations. 2. All bind to at least one molecular target. Highly specific. 3. All perform at least 1 cellular function. |
|
|
Describe the structure of proteins |
The positively charged N-terminus is where the amino group (NH2) is. The negatively charged C-terminus is where the carboxy (COOH) is. These create the protein backbone.
The alpha carbon sits in the middle
The R group (or side chain) determines the type of amino acid |
|
|
What kinds of bonds connect amino acids? |
Peptide bonds, which are catalyzed by the ribosome during translation. |
|
|
How many classes of amino acids are there, and what determines the class?
See Pp for images of each side chain type. |
The Side chain (R group) determines the class.
1. Nonpolar, aliphatic side groups. Ex: Methionine 2. Aromatic side groups. Ex: Phenylalanine 3. Polar, uncharged side groups. Ex: Threonine 4. Positively charged side groups. Ex: Lysine 5. Negatively charged side groups. Ex: Glutamate |
|
|
What are the 4 levels of protein structure? |
1. Primary: aa sequence 2. Secondary: Alpha helix, beta pleated-sheets, and random coils. Based on the primary structure, but not easily predictable 3. Tertiary: The 3D conformation of the protein. Local structure: motifs and global/overall structure. 4. Quaternary: When the protein molecule is formed as a complex of more than one polypeptide chain |
|
|
What is the tertiary local structure? |
The combo of several individual secondary structures together into a characteristic motif structure seen in many proteins. Ex: helix-loop-helix motif and hairpin turn motif (2 head-to-tail beta-sheets). |
|
|
What is tertiary overall structure? |
The shape of the entire protein for a single polypeptide. |
|
|
What are different models used to depict secondary, tertiary, and quaternary structure? |
1. Ribbon model (makes it easy to see alpha helices and beta sheets) 2. Space-filling model (emphasizes overall shape) 3. Backbone model |
|
|
How many types of overall tertiary structure are there? |
Two: globular and fibrous (long rod-shaped proteins made from parallel, cross-linked polypeptide chains) |
|
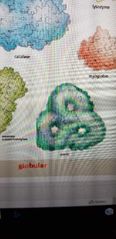
What protein is this? |
Aspartate transcarbamoylase. Globular structure |
|

What protein is this? |
Catalase. Globular structure |
|
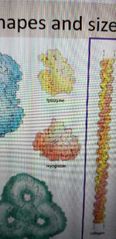
What are these two proteins? |
Yellow one is lysozyme Red one is myoglobin Globular structures |
|
What protein is this? |
Porin. Globular structure |
|
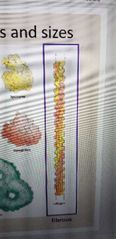
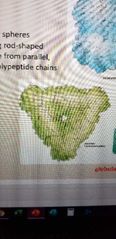
What is this protein? |
Collagen. Fibrous structure |
|
|
How is the quaternary structure defined? |
By arrangement of polypeptide subunits in multimeric proteins. Ex: Antibodies, which have two light chains connected by disulfide covalent bonds to two heavy chains |
|
|
What are some of the quaternary structures? |
Homodimers, helices, rings, spheres, and hollow tubes. |
|
|
What are the 5 types of chemical bonds that fold and stabilize protein structure? |
1. Disulfide bond. Only covalent bond, and by far the most stable. Non-covalent 2. Hydrogen bond. Occur with alpha helices and beta sheets. 3. Electrostatic interactions. Can neutralize charged residues when they occur in the interior, which makes interior hydrophobic. 4. Hydrophobic interactions. Usually occur in the interior of protein, leaving polar/ionic residues at surface to interact with water. 5. Van der Waals forces. Only act at very short distances. |
|
|
True or false, reorganization of a few non-covalent bonds allows proteins to switch conformations? |
True |
|
|
What was the Anfinsen in vitro experiment? |
A test using Ribonuclease A to see if all necessary information for proper folding of a protein is contained in its primary sequence. Proved that yes, it does. |
|
|
How do cells fold proteins so quickly? |
Chaperone proteins. Some prevent misfolding by destabilizing weaker conformations using energy of ATP hydrolysis. Others create chambers to isolate nascent proteins. |
|
|
What do proteins need to be functional? |
They must be able to be activated and inactivated. The two states are reflected by different conformations. |
|
|
How many mechanisms (at least) are there for controlling protein activity? |
1. Chemical modification of amino acids 2. Binding of small molecules to allosteric sites 3. Binding of regulatory proteins 4. Protein degradation |
|
|
What are the 7 common types of covalent protein modifications? |
1. Disulfide bonds (Cystine) 2. Ubiquitination (Lysine) 3. Sugars (glycosylation, Asparagine, serine, threonine) 4. Lipids (ex: farnesyl, cystine) 5. Phosphate groups (phosphorylation, tyrosine, serine, threonine) 6. Methyl groups (methylation, lysine, arginine) 7. Acetyl groups (acetylation, lysine) |
|
|
True or false: protein modifications do not depend on context (surrounding amino acids). |
False. They do depend on context, and because of this, only a few particular residues undergo modifications. |
|
|
True or false: many chemical modifications of proteins are not reversible. |
False. Proteins can switch between active and inactive many times. |
|
|
With small molecule modifications, why are active or inactive states relatively short lived? |
Proteins bind and release small molecules extremely quickly from allosteric sites. Costs the cell a lot of energy to maintain a steady supply of them. |
|
|
What is an allosteric site? |
The place on an enzyme where a molecule that is not a substrate may bind, thus changing the shape of the enzyme, affecting whether it's active or inactive. |
|
|
What is the only non-reversible protein regulatory mechanism? |
Protein degradation |
|
|
How can protein expression levels be regulated? (Meaning at what levels) |
Transcription, translation, and protein stability |
|
|
What are the 3 degradation mechanisms? |
1. Proteasomes (source is nucleus and cytosol) 2. Lysosomes (source is plasma membrane and Golgi apparatus) 3. Soluble proteases (source is extracellular matrix/ECM) |
|
|
What is the signal for protein degradation by the proteasome? |
A poly-ubiquitin tag. |
|
|
Describe the 20S proteasome structure. |
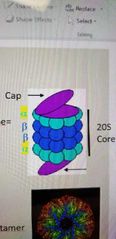
It has a cap on either end, which unfolds targeted proteins. The core is made of two alpha and two beta units in this order: abba. These rings are each 7 proteins. The target protein is degraded within these rings (which create the core). |
|
|
What do enzymes do? |
Bind substrates and convert them into products by speeding up a chemical reaction. They are catalysts. |
|
|
What are motor proteins? |
Proteins that generate force to move cells or cellular components within cells. |
|
|
What are response proteins? |
They detect signals inside or outside of cells and transduce these signals to activate cellular changes. Often regulate other proteins or gene expression. |
|
|
What are structural proteins? |
They provide scaffolds for the cell or for structures within the scaffold. |
|
|
What are transport proteins? |
They move material in/out and within the cell. |
|
|
True or false: proteins work in complexes |
True |
|
|
What holds protein complexes together? |
Multiple non-covalent bonds between protein surfaces. They are weak individually, but strong when there are many of them. |
|
|
What determines how a protein works? |
Side chains and spatial organization. Side chains don't need to be next to each other to bond. |
|
|
What is X-ray crystallography, and how does it work in general? |
A way of determining protein structure. 1. Purify single protein 2. Crystallize protein 3. Obtain X-ray diffraction pattern |
|
|
What is SDS PAGE and how does it basically work? |
A method for separating proteins based on size.
1. Separate proteins of different size using sodium dodecyl sulfate and polyacrylamide gel in gel electrophoresis. 2. Follow up with a western blot because cells have so many proteins, need some way to distinguish them. SDS has a hydrophobic tail with a negative charge, coating the protein to maintain a denatured shape so shape doesn't affect test |
|
|
What is a Western Blot? What is the procedure? |
A procedure used to detect a specific protein from a mixture of proteins. Uses antibodies as probes. 1. Dissolve and isolate proteins in Sodiumdodecyl Sulfate and then boil (helps unfold proteins and coats them with a negative charge for gel electrophoresis)2. Separate protein by SDS-PAGE (Sodiumdodecyl Sulfate - Polyacrylamide gel electrophoresis). PAGE works better for proteins than agarose.3. Transfer proteins onto nylon membrane. 4. Add primary antibody that binds to protein of interest. Wash off excess. 5. Add secondary antibody, which binds to primary, and is covalently linked to a report enzyme. 6. Add enzyme substrate linked to secondary antibody to produce color or luminescence. 7. Expose to X-ray film to find target protein. |
|
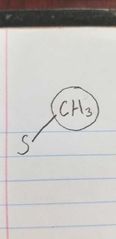
Name the class of amino acid and an example. |
Nonpolar, aliphatic side group Methionine. |
|
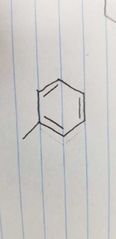
Name the class of amino acid and an example. |
Aromatic side group Phenylalanine |
|
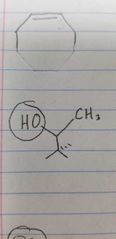
Name the class of amino acid and an example. |
Polar, uncharged side group Threonine |
|
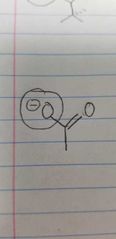
Name the class of amino acid and an example. |
Negatively charged side group Glutamate |
|
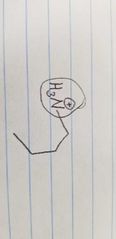
Name the class of amino acid and an example. |
Positively charged side group Lysine |
|
|
Describe the amino acid peptide bond formation |

|
|
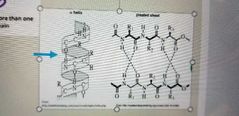
What are these two structures? |
1. Alpha helix 2. Beta pleated sheet Both are secondary protein structures. The third major 2⁰ structure is the random coil |

