![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
4 Cards in this Set
- Front
- Back
|
Explain how the electrons in an atom are arranged. |
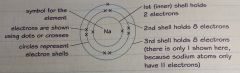
The electrons in an atom are arranged in electron shells, or energy levels around the nucleus. The electrons fill the shells that are closest to the nucleus first. The diagram shows the electronic configuration of sodium. This can also be written in numbers: 2.8.1 |
|
|
The atomic number of potassium is 19. Explain how its electrons are arranged. |
An atom has the same number of protons and electrons, so a potassium atom has 19 electrons. The inner shell fills up first, and this shell is full when it has 2 electrons. The second shell has 8 electrons, and this is also full. This leaves 9 electrons. The third shell can hold 8 electrons, so that is full as well. The last shell has 1 electron in it. We write this as 2.8.8.1 |
|
|
Explain how the group numbers in a periodic table relate to the number of electrons in shells. |
The group numbers are the same as the number of electrons in the outer shells (apart from group 0, where the outer shells are full) |
|
|
Explain how the period number relates to the shells of an element. |
The number of occupied shells is the same as the period number. |

