![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
2 Bonding, 0 Lone pairs |
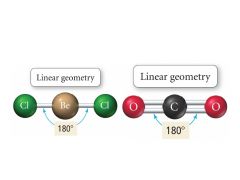
Electron: Linear |
|
|
3 Bonding, 0 Lone pairs |
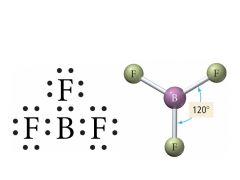
Electron: Trigonal Planar |
|
|
2 Bonding, 1 Lone pair |
Electron: Trigonal Planar |
|
|
4 Bonding, 0 Lone pairs |
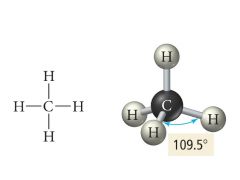
Electron: Tetrahedral |
|
|
3 Bonding, 1 Lone pair |
Electron: Tetrahedral |
|
|
2 Bonding, 2 Lone pairs |
Electron: Tetrahedral |
|
|
Electron Geometry for 2 Electron Groups |
linear 180° bond angle |
|
|
Electron Geometry for 3 Electron Groups |
trigona planar 120° bond angle |
|
|
Electron Geometry for 4 Electron Groups |
tetrahedral 109.5° bond angle |

