![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
|
Range of resting membrane potential |
-40 to -90 mv |
|
|
Microelectrode structure |
Piece of glass tube with a conductor inside. Contains a small opening which allows for measurement of transmembrane voltage. |
|
|
Receptor potential example |
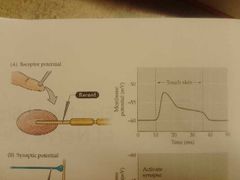
Pacinian corpuscles; create receptor potential upon touch of skin |
|
|
The intensity of a stimulus is encoded in the... |
frequency of action potentials |
|
|
Passive vs active |
Does it depend on cell function or not? Does it decay over distance, or not? |
|
|
Concentration vs electrochemical gradient |
A 10x increase in concentration gradient in KCL ions is counterbalanced by the movement of 10^-12 moles of K+ ions per square centimeter of membrane. In this instance that refers to a millionth of the K+ ion concentration. Thus in this instance the electrochemical gradient is million times more powerful than the concentration gradient. |
|
|
What is R? |
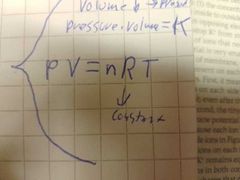
|
|
|
Nernst equation |
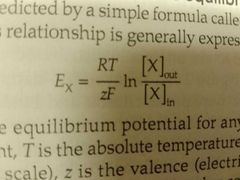
Predicts the difference between inside and outside compartments at given concentrations of valent ion. More precisely, predicts a -58mV decrease in voltage for every tenfold increase in univalent ions, and half as much for divalent etc. I speculate that the reason concentration isn't taken into account is because the effect is neglecteable. |
|
|
Goldman equation |
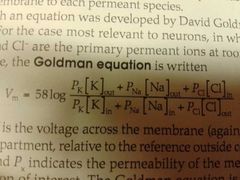
Takes into account two things then Nernst equation did not 1) permeability, described by px 2) Presence of multiple, though univalent ions |
|
|
Hodgkin and Katz (1949) |
By proving the principles behind the Nernst and Goldman equation through using huge giant squid neurons, they found that manipulating K+ ions has the largest effect, and this concluded the membrane to be most permeable to these ions. Additionally, they found that some mysterious effect causes there to be 200 times more K+ ions within the cell than outside of it. Furthermore they did find that the same logarithmic principles apply that were found along with the Nernst equation. |
|
|
Hodgkin and Katz; Na+ escapades. |
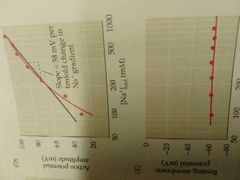
Found that the amplitude of an action potential decreases as external NA+ concentration is modified. The logarithm of the external NA+ concentration is linearly related to the amplitude of action potential. Thus the specific role of the NA+ ions was found. |

