![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
75 Cards in this Set
- Front
- Back
|
What are the two major applications for stable isotope geochemistry in the earth sciences? |
1. Thermometry: Looking at the formation temperature of rock, corals, ect We can use this to determine a paleotemperature scale for the reconstruction of environments.
2. Tracers: Different ratios of stable isotopes exist in different lat/long/ elevations on Earth. We can use isotope signatures to look at where things came from |
|
|
What is an isotope? |
Atoms that have the same number of protons (z), but a different amount of neutrons (n) mass (A)= (8)+(8) = 16. |
|
|
What is a stable isotope? |
A non radiogenic atom with the same atomic # but different n
Most elements have more than 1 stable isotope but there are 21 elements that are monoisotopic |
|
|
What is an isotopologue? |
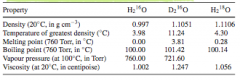
All the isotope variations of a molecule. For example, the isotopologues of water are all made up of different combinations of the different isotopes of H2 (HH, DH, DD) and O (16O, 17O, 18O).
Different isotopologues have different vapour pressures (it is easier to evaporate a water that has weaker bonds therefore it has a higher vapour pressure and will be enriched in the vapour phase) |
|
|
T/F: The less mass there is in an isotope, the more unstable it is because the bond strengths are weaker. |
True! |
|
|
What does the symmetry rule say about the stability of nuclides? |
In a stable nuclide with a low atomic # (<20), the # of protons is approximately equal to the # of neutrons (N/Z is approximately equal to 1).
Once you get over Z= 20, it no longer follows an exact N/Z=1 line (Figure 1.1).
In stable nuclides with >Z=20, the N/Z ratio is always greater than N:Z=1. I.e, 202Hg Z=80 and N= 122 |
|
|
What is the Oddo-Harkins rule? |
Nuclides of even atomic # are more abundant than those with odd numbers (has to due with symmetry rule.. even nuclides are more stable so therefore more abundant)
|
|
|
What is an isotopic ratio? |
Isotopic ratios of the elements are written conventionally as the ratio of the heavy isotope to the light isotope ALWAYS WRITE (HEAVY/LIGHT)
i.e, R= D/H, 18O/16O, 34S/32S, 13C/12C , 11B/10B, 56Fe/54Fe
|
|
|
What is the EQ to get δ values from R sample and R std values? |
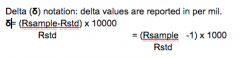
We want to get the ratio of the isotope in the sample and divide it by the ratio of isotopes in the standard so that we can see the relative abundance of the heavier isotope in the sample. Using δ 18O as an example, If we compare the sample to the standard we can see if it was hotter/wetter (enriched in δ18O) or if it was colder/ dryer (depleted in δ18O) at the time of formation. |
|
|
Why do we use δ notation? |
It makes more sense to use δ notation when we are looking at isotopologues because it is much easier to see the ratio value (Lec1, SL28) when there are small variations in values |
|
|
What is the EQ for fractionation factor (α)? |
The isotopic fractionation factor between two substances A and B is defined as: Ra Rb
|
|
|
What are the standards for: |
Hydrogen: V-SMOW (standard mean ocean water), VSLAP, GISP, NBS 30 |
|
|
What are the four principles of the mass spec? |
1. Ion source: ions are formed, accelerated, focused into a narrow beam. A beam of electrons is emitted by a heated filament and bombards the electrons to ionize them. Any positively charged molecules are accelerated by a high voltage potential to be injected into the magnetic field.
Positively charged ions pass through a strong magnetic field that deflects the ions in a circular trajectory according to the ratio of mass to charge. Physical separation between the ions of different isotopologues
|
|
|
How does the magnet in the mass spec differentiate the weights of different isotopes? |
Positively charged ions pass through a strong magnetic field that deflects the ions in a circular trajectory according to the ratio of mass to charge. Physical separation between the ions of different isotopologues.
The heavier the ion, the larger the radius will be (takes more force to deflect it relative to the light ions) Light ions are deflected more strongly than heavy ones of the same charge. The radius of the ion’s path is equal to the root of the mass. i.e, out of all of the CO2 isotopologues, 44C will have the smallest radius (46C will have the largest mass and largest radius=more deflection)
This allows us to physically separate and then count the relative abundance of isotopes in the sample (relative to the standard)
|
|
|
What is CF-IRMS? |
CF-IRMS: Sample inlet gas via carrier gas, no pressure adjust, linearity and stability of system are necessary conditions, one peak per sample
Advantages of CF IRMS 2. Rapid analysis 3. Sample sizes are reduced by several orders of magnitude *Precision is not as accurate as dual inlet system
|
|
|
What is the δ value of sea water? |
0! |
|
|
True/ False: The α fractionation factor is dependent on temperature |
YES! |
|
|
What are common hydrogen fractionation processes? |
1. Phase transitions of water between vapour, liquid, ice |
|
|
What is the Global Meteoric Water Line (GMWL) EQ? |
δD= 8(δ18O)+10
|
|
|
What are the stable isotopes of Boron? |
10B and 11B |
|
|
What are the four major climate archives? |
1. Ice cores |
|
|
What are the four working hypotheses needed for B isotope paleo pH proxy? |
The relative proportion and thus the δ11B value of boric acid B(OH)3 and borate ion B(OH)4- in seawater is a fn of pH (figure 2.8 in Hoefs) The tetrahedrally coordinated borate ion is depleted in 11B relative to boric acid at equilibrium borate ion B(OH)4- is preferentially incorporated into marine carbonates without a significant kinetic isotopic effect As a result, the pH of seawater determines the δ11B of marine carbonates |
|
|
True/False:
at a pH of under 7, the relative abundance of boric acid is much higher at a pH of over 7, the borate ion is more abundant |
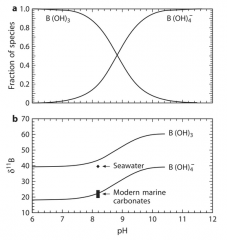
True |
|
|
What are the stable isotopes of C? |
12C and 13C 2. 16th most common element in Earth's crust 3. A major element of life 4. Occurs as CO2, HCO3, and carbonate minerals |
|
|
True/ False: photosynthesis is the second most important fractionation process for C |
False! |
|
|
What isotopologues are commonly used to measure δ value of C?
|
CO2 and CO
Measure 45/44 for CO2 Measure 29/28 for CO In the most simple cases, we can ignore the isotopologues that have a very tiny abundance (we can/ should still take the other isotopes into account) |
|
|
How are the δ values of C obtained from organic matter? |
living vegetation or its organic residue in soils, organic matter in plankton + ocean sediments are oxidized at high T (850-1000 deg) in a stream of oxygen or in the presence of an oxidizing agent (such as CuO) to produce CO2.
Measure C isotope composition of CO2 of burned up plants + things |
|
|
What are the most popular materials for C δ value analysis? |
Carbonates and organic compounds |
|
|
True/ False: During glacial times there is a low amount of d13C in the oceans and the atmosphere
|
True! (why?) Ocean surface waters exchange all their C with the atm in just a few years |
|
|
What are the stable isotopes of N? |
14N and 15N
Largest single constituent of the Earth’s atmosphere 14N more abundant 3. Standard = AIR (atmospheric nitrogen) There is a loss of light N2 to the atmosphere, creating a positive 15N value in ocean water |
|
|
What is the difference between N isotopic composition in artificial and natural fertilizers? |
artificial= -4 to 4 permil (made from atm nitrogen) organic (+6 to +30 permil) comes from N15 enriched animal waste We may be able to differentiate two different types of fertilizers by looking at their isotopic signatures + ranges |
|
|
What are the 5 N reservoirs on Earth? |
1. Atmospheric N 2. Plants 3. Nitrates 4. Nitrites 5. Ammonium |
|
|
What are the fractionation processes of the N cycle? |
1. aquatic -27 to 0 permil |
|
|
T/F: Denitirification is the most intense in poorly drained or poorly oxidized soil because nitrate consuming organisms become active only when O levels are low
|
True!
Isotopically light nitrogen will be enriched in the lighter value of N via denitrification
The loss of N2 increases the d15N value of any remaining nitrate remaining source becomes isotopically heavier and heavier
|
|
|
What isotopologues of N do we use to measure the δ of N2 gas? |
Measure 29/28 Measure 30/28 only when materials are artificially enriched in 15N |
|
|
Kinetic vs. EQ Isotope Effects |
Kinetic Isotope Effects: Non reversible, associated with unidirectional and incomplete processes - Often very large fractionations - EX: evaporation, dissociation, biological rxns. ect EQ Isotope Effects: Reversible - Fractionations generally small - EX: high T isotope fractionation, long term phase interactions |
|
|
Equilibrium Isotope Effects |
The effect of atomic mass on bond energy
Heavier atoms will have a more intense bond strength (require more energy to break bonds)
It will take more potential energy to break the bonds of the Hydrogen ion deuterium (DD) than protium (HH) because the masses are heavier. |
|
|
What is zero point energy? |
the energy of an atom at absolute zero, or the ground vibrational state
|
|
|
What are two reasons why H isotope geochemistry is cool? |
1. Hydrogen is EVERYWHERE in terrestrial environments 2. The relative mass difference between H and D is large so we get large fractionations
Because of EQ Isotope Effect, we can use D concentrations in samples to look at variation in RH (because when it is more humid there will be more D relative to H) |
|
|
Lab analytical techniques for Carbon |
Reacted with phosphoric acid at temperature between 25-90 |
|
|
Fractionation processes for C
|
1. EQ isotope exchanges with inorgani carbonate systems (at 25 deg, the carbonate ion HCO3 is the heaviest then CO3 and H2CO3)
2. Kinetic isotope effects in psynth - C3 and C4 plants fractionate C differently (this signature can be used to look at what kind of plants were happening) |
|
|
Why is there low 13C in the oceans during glacial times?
|
There are not really any plants on land cause it is cold as tits, therefore there is nothing to preferentially fractionate and take out 12C from the atmosphere therefore the ratio of 12C is higher.
The opposite occurs in interglacial times because there is a lot of plants to take 12C and then 12C gets depleted while 13C is enriched |
|
|
What are the major fractionation processes for O?
|
1. Evaporation
2. Kinetic effects (RH of air, turbulence in liquid) 3. Fractionation among O bearing minerals |
|
|
T/F: Aragonite and calcite have the same delta value since they are both calcium carbonate.
|
FALSE! They have roughly different delta values based on many properties
|
|
|
What are common geologic samples
|
1. Water
2. Carbonates 3. Silicates/ Oxides 4. Phosphates 5. Sulfates |
|
|
How many stable isotopes does Sulfur have?
|
4!
32, 33, 34, 36 |
|
|
What is the sulfur isotopic composition of ocean water?
|
21 permil
|
|
|
How is sulfur fractionated in nature?
|
1. Kinetic isotope effect of psynth
2. Dissimilarity S reduction - The major process of changing sulfate to sulfide -anaerobic process -energy yielding reactionbacteria use sulfate as an electron acceptor |
|
|
How does the reduction of S impact isotope fractionation?
|
There is an inverse relationship between S fractionation and reduction. Low reduction (dry): higher fractionation (-20 to -46) |
|
|
What are two S gases commonly used in IRMS?
|
SO2
SF6 SF6 has no mass spec memory effect because F is monoisotopic. But I think it is rather dangerous because flourine is reactive |
|
|
What are "neat facts" about Calcium?
|
1. plays an essential role in biological processes
2. T dependence of Ca isotope fractionation (paleothermometer temperature) 3. Marine biogenic carbonates depleted in 44Ca |
|
|
List some non traditional stable isotopes
|
Ca
Li Fe MgCl |
|
|
What are the two most abundant stable isotopologues of water?
Which ones are abundant enough to use in stable isotopes? |
most common: HH16OHD16O HH16O HH18O HH17O HD16O/DH16O |
|
|
What is meteoric water?
|
Liquid or solid water that falls from the sky.
- resides on earth principally as glaciers, aquifers, rivs, ect The GMWL looks at the covariance of d18O and dD of meteoric water |
|
|
What is a closed system (isothermal) equilibrium process?
|
Isothermal =temperature does not change, no in and out of box
- the condensed liquid and remaining vapour are always in contact with each other and in equilibrium |
|
|
What is a closed system (non isothermal) EQ process?
|
Closed system (non isothermal… aka temperature will drop.. this is more similar to nature because as air masses rise their T drops)
when the T of an air mass decreases to the point of supersaturation, condensation and precip will occur -The non isothermal model is therefore a better example of nature |
|
|
How far back do the climate signals of the Antarctic + Greenland go back?
|
Antarctic = 800 ky
Greenland = 125 ky |
|
|
How is the isotopic signature of the ocean changed?
|
Evaporation will increase d18O value
- differences in precip/ runoff input will change composition |
|
|
What is (roughly) the d18O value of atmospheric O2?
|
~23.5 permil
|
|
|
What do long term measurements of CO2 concentration in ice cores indicate?
|
atm Co2 increases by about 1.5 ppm per year
d 13C of CO2 shifts towards lower values |
|
|
The observed vs calculated shift in CO2 is smaller that we thought b/c:
|
50% of the CO2 emitted into the atm remains
other 50% is absorbed into the oceans + terrestrial |
|
|
Why is there a seasonal d13C record?
|
1. Respiration from plants
2. There is a large seasonality from the respiration of plants in the N.H 3. There is more land in the N.H then in the south |
|
|
The differences in the isotopic composition of C4 and C3 plants help us with what?
|
1. Paleoclimate indicator
2. Diets of animals (roughly) |
|
|
How is H fractionated in plants?
|
H enters the plant as water without any subsequent isotopic fractionation
water transpiration is associated with large -positive isotope fractionation (+ 40 or 50 permil) -large negative isotope fractionations in the biochemical reactions |
|
|
How is O fractionated in plants?
|
CO2 H2O and O2 are the main sources of O for plants
controlled experiments have shown that d18O of water mostly determines the d18O of a plant or organically bound oxygen No apparent fractionation during the uptake of soil water |
|
|
How does d13C vary among marine phytoplankton?
|
d13C of marine phytoplankton varies up to 15%
-there are different latitudinal trends between the northern and southern oceans -strong correlation in south ocean weaker relationship in north ocean A significant inverse relationship (SO) high latitude 13C depletion of phytoplankton[CO2] in surface waters paleoCO2 concentrations vs. d13C of marine phytoplankton? |
|
|
What does "you are what you eat +/- a few permil"
|
the d15N composition of animals is related to their diet!
- d15N increases by 3-4% for each successive trophic level d15N of animals is generally greater than the d15N food it eats - not all tissues have the same value 15N of urinary urea is 2-4% more negative than the diet YOU CAN USE THE 15N/13C ratio in the diets of people or animals! high 15N= carnivorous diet (high trophic level) low 13C= lots of C4 plants (probably corn) |
|
|
What are the differences in C fractionation between marine + terrestrial plants? Why is this important?
|
7 permil difference in 13C
- can be used to trace the input of terrestrial/oceanic matter C isotopic fractionation associated with the production of terrestrial organic matter has remained relatively constant C isotopic fractionation associated with the production of organic matter has changed over geologic time (variation in dominant biogeochemical processes/ environmental conditions ) |
|
|
T/F: coal and fossil fuel have a lot of 13C
|
NO it is very depleted (since it is made up of dead plants which mostly take in 12C)
|
|
|
Oxygen isotope thermometry
|
the fractionation between coexisting minerals decreases with (increasing) T
Two phases can be used to determine the T of metamorphism and formation of rock, mineral, and gas systems |
|
|
Carbonate clumped isotope thermometry
|
If we know the d value of the carbonate and water, we can use these to back-calculate formation T
|
|
|
What are "vital effects" and why are they important?
|
Effects of living things..
1. these microorganisms all grow at different rates (incorporate different amounts at different times) 2. water chemistry is anisotropic and therefore different life modes will have biased isotope values based on where they were living in the water column and also where in the world they are Planktic forams live vertically dispersed in the upper water columns O isotopic composition from deep ocean water benthic forams may be more accurate because the T at the bottom of the ocean does not change much Planktic forams are impacted by ocean T and ice sheets |
|
|
What are the factors that influence the isotopic composition of water?
|
1. Temperature
2. Amount 3. Continental/coastal 4. Altitude 5. Season 6. Latitude |
|
|
Natural origins of sulfur
Anthropogenic origins of sulfur |
Natty:
1. Volcanics 2. Sea spray 3. aeolian weathering 4. biogenic Anth: 1. combustion of fossil fuels 2. ore smelting 3. gypsum processing |
|
|
What is the d18O variation in forams caused by?
|
1. Change in ocean water T
2. Change in isotopes of ocean (i.e, d18O, salinity, ect) |

