![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
155 Cards in this Set
- Front
- Back
|
Metformin |
inhibits hepatic gluconeogenesiso Weight neutral or can induce weight loss, low cost and long track recordo GI adverse effects (nausea, loose stool, flatulence), lactic acidosis (renal impairment, heart failure, liver disease), unsafe if GFR<30
|
|
|
Sulphonylureas
|
Sulphonylureas increase insulin secretion but can cause hypoglycaemia and weight gain
|
|
|
Thiazolidenediones
|
Eg. pioglitazone enhance lipogenesis, decrease lipolysis and decrease plasma FFAs (PPAR-γ agonists), but may cause fluid retention and weight gain and increase peripheral fracture rate. They may have an association with bladder cancer and are now rarely used, mostly in NAFLD
|
|
|
DPP-IV inhibitors
|
Eg Sitagliptin Inhibits the DPP-IV enzyme which breaks down GLP-I (Incretin). Therefore DPP-IV inhibitors prolong the incretin activity Well-tolerated, don’t cause hypoglycaemia, some licensed for use at low GFR. High cost, long term effects uncertain |
|
|
GLP-I mimetics |
Eg. exenatide, agonists of the GLP-1 receptor
Are resistant to DPP-IV, so enhance incretin effects. No hypoglycaemia, weight loss. SC injection, nausea and vomiting, long term effects unknown, high cost |
|
|
SGLT2 inhibitors
|
Sodium/ glucose cotransporter 2 inhibitor Prevent renal glucose reabsorption in the PCT, causing weight losso can cause UTIs, is high cost with uncertain long term effects |
|
|
NPH |
Intermediate acting insulin eg. Isophane insulin |
|
|
Human insulin |
made using recombinent DNA |
|
|
Insulin analogs |
Based on human inslulin but slight tweeks have been made to make it more effective. Therefore more of an insulin receptor ligand. These are useful as they reduce nocturnal hypos and allow for lower fasting glucose |
|
|
Constant infusion rapid acting analogue
|
Allow more predictable absorption and smaller SC volume. Basal rate with meal-time boluses reduces hypoglycaemia but active self-management with high incidence of DKA if not used properly and high cost
|
|
|
Statins |
By inhibiting HMG-CoA reductase, statins block the pathway for synthesizing cholesterol in the liver. This is significant because most circulating cholesterol comes from internal manufacture rather than the diet. When the liver can no longer produce cholesterol, levels of cholesterol in the blood will fall
Serious side effects are rare; myositis or myopathy, hepatitis, with increased risk in renal impairment, hypothyroidism and drug interactions |
|
|
Criteria for diagnosis of DKA |
ketonaemia >3mmol/l or significant ketonuria (>2+ on dipstick)
BM>11 or known DM bicarbonate <15mmol/l or venous pH<7.3 |
|
|
What is the management of DKA? |
-500ml 0.9% saline rapidly in DKA; can give up to a litre
-Begin potassium replacement early in DKA in adults, with frequent monitoring for the development of hypokalaemia. -Insulin should be administered via short-acting insulin IV infusion at 0.1unit/kg/hour using infusion pump it should be mixed with saline -Commence glucose alongside insulin when patient more alert with lower ketones |
|
|
What regular investigations should be undertaken for a patient in DKA? |
Hourly BM, hourly ketones, venous bicarbonate and potassium 2 hourly, 4 hour plasma electrolytes
|
|
|
Define the resolution of DKA |
Resolution is defined as blood ketones <0.3mmol/l and venous pH>7.3
|
|
|
Once DKA resolution has been confirmed how is management stopped? |
fast-acting insulin SC with meal and discontinue insulin infusion 30 minutes later
|
|
|
How should a hypo be managed? |
If IV access in unconscious patient, give 75-80ml 20% dextrose or 150-160ml 10%. If no IV access, give Glucagon 1mg IM, recheck in 10 minutes, and repeat if below 4 Give long-acting oral carbohydrate after immediate recovery
|
|
|
Define Pharmacogenetics |
The study of the genetic basis for variability in drug response
|
|
|
Pharmacogenomics
|
The use of genetic information to guide the choice of drug and dose on an individual basis; for personalised therapy
|
|
|
Describe the effects of a Polymorphism in the TPMT gene |
Polymorphisms in the TPMT gene result in decreased TPMT enzyme activity, reducing breakdown of the thiopurine drugs used to treat acute lymphoblastic leukaemia and increasing risk of severe bone marrow toxicity
|
|
|
Give some examples of idiosyncratic (bizarre) drug reactions which now seem to be associated with genomics |
SJS due to carbamazepine, hepatitis with flucloxacillin, aplastic anaemia with chloramphenicol and rhabdomyolysis with simvastatin
|
|
|
What is the basic diagnostic criteria for PD? |
characterised by bradykinesia with at least one of rigidity, resting tremor and postural instability
|
|
|
How are abnormal proteins involved in PD |
abnormal protein aggregations of α-synuclein often collected into intracellular inclusions (Lewy bodies)
|
|
|
Co-careldopa
|
The combination of the two medications carbidopa and levodopa. Levodopa is a precursor of dopamine with a 60 minute half-life, given with dopa-decarboxylase inhibitor, which degrades dopamine peripherally)
|
|
|
Levadopa |
-L-DOPA crosses the protective blood–brain barrier, whereas dopamine itself cannot. Thus, L-DOPA is used to increase dopamine concentrations.
-SE's: postural hypotension, insomnia, vivid dreams, nightmares, confusion, visual hallucinations, delusions, nausea, vomiting --Abnormal involuntary movements (dyskinesia) and end-of-dose deterioration in the long term -Consider fractionating dose or giving adjuvant treatment |
|
|
Carbidopa |
Dopa-decarboxylase inhibitor. A drug given to people with Parkinson's disease in order to inhibit peripheral metabolism of levodopa. This property is significant in that it allows a greater proportion of peripheral levodopa to cross the blood–brain barrier for central nervous system effect.
|
|
|
Dopamine agonists
|
Eg ropinirole. Act directly on the post-synaptic dopamine receptors in the striatum; with oral, transdermal, or SC administration. Similar side effects with impulse control disorders; gambling, hypersexuality, compulsive buying, binge eating |
|
|
Describe the management pathway for patients with PD |
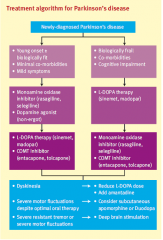
|
|
|
MOA-B inhibitors
|
selegiline irreversibly inhibit monoamine oxidase B, reducing breakdown of dopamine so more remains in the synaptic cleft
Possibly neuroprotective so used first line in younger patients Nausea, vomiting, confusiono Potential interaction with SSRIs, may cause serotonin syndrome |
|
|
COMT inhibitors
|
(entacapone) inhibit catechol-O-methyltransferase so more levodopa is available to cross BBB for prolonged effect
diarrhoea, discolouration of body fluids, may increase dyskinesia |
|
|
Amantadine
|
An antiglutaminargic and NMDA receptor antagonist, which increases dopamine release, used as an adjuvant treatment in late PD to treat dyskinesia o confusion, hallucinations, psychosis, ankle oedema
|
|
|
Anticholinergics in PD
|
only used in young patients with a prominent tremor, reducing effects of relative central Ach excess that occurs due to dopaminergic deficiencyo May cause cognitive impairment in elderly, dry mouth, constipation, dizziness, blurred vision, urinary retention, glaucoma
|
|
|
What are the general principles for management of epilepsy |
-Generally, valproate used for generalised; carbamazepine (+ lamotrigine) for partial
-Lamotrigine or levetiracetam in women of child-bearing age for generalised |
|
|
What is the management of absent seizures? |
Treated with ethosuximide or lamotrigine if not tolerated.
|
|
|
Describe the adverse drug effects associated with anti-convulsants |
-Anti-epileptic drugs can cause acute allergic reactions with a skin rash, or toxic effect reactions with unsteadiness, blurred vision, tremor and confusion
-hair loss, overgrown gums, memory loss, mood change, anaemia, osteomalacia -Valproate associated with Parkinsonism, weight gain, teratogenicity (spina bifida) |
|
|
Describe the problems that epileptic women face when using long term contraception |
Lamotragnine, Phenytoin and Carbomazapine are CYP450 inducers, therefore they speed up the breakdown of OCP, so be careful. Does for the COCP should be at least 50 micrograms a day. Implant, POP, patches should be avoided |
|
|
Describe the management of status epileptics |
Secure airway, give oxygen, take collateral history, gain IV access, check glucose + thiamine IV lorazepam 0.07mg/kg or 4mg or diazepam 10-20mg, up to 40mg; repeat after 5 minutes Phenytoin 15-18mg/kg after 20-30 mins; 0.5-1g if already on
|
|
|
What are the risk factors for SUDEP? |
uncontrolled epilepsy, young age, generalised seizures, learning disability, seizures in sleep, unwitnessed seizures, and poor compliance.
|
|
|
What is the management of Delirium Tremens |
Give chlordiazepoxide PO (benxodiazapine used in alchohol withdrawl) as needed based on symptom score, and IV thiamine for three days
|
|
|
Describe the ADR's of Carbamazopine |
-can cause drowsiness at first but it wears off
-hypersensitivity, mouth ulcers, lymphadenopathy, fever, neutropenia -toxicity causes double vision, unsteadiness (cerebellar signs); reduce dose, supportive -γ-GT can be raised as it is an enzyme inducer; contraindicated with Warfarin, can induce its own metabolism -hyponatraemia as it causes SIADH; only discontinue if symptomatic |
|
|
Describe the common symptoms of drug induced parkinsonisism? |
bilateral (disease unilateral initially) subacute onset, early presence of postural tremor, with progression concurrent with medication intake.
|
|
|
What drugs are likely to lead to drug induced parkinsonism? |
With any dopamine antagonists, such as anti-emetics (metoclopramide, prochlorperazine) as well as haloperidol, risperidone, valproate, lithium, calcium channel blockers and SSRIs such as fluoxetine
|
|
|
List the main hepatotoxic drugs |
Paracetamol, isoniazid, statins methotrexate, phenytoin, aspirin, alcohol, antibiotics, and oral contraceptives
|
|
|
Describe hepatic drug clearance |
-The volume of blood perfusing the liver that is cleared of the drug per unit of time, dictated by blood flow through the liver (Q) and:
-Fraction of the drug that is free of unbound plasma proteins and capable of interacting with hepatic enzymes (f) -intrinsic clearance (Cl), the ability of the liver to metabolise the drug in absence of flow limitations and protein binding |
|
|
What is the hepatic extraction ratio? |
The percentage of the drug removed from the perfusing blood during passage through the organ is the extraction ratio; the ratio of hepatic clearance to hepatic blood flow
|
|
|
What does a 'large first pass effect mean'? |
Drugs with high hepatic Extraction ratio have a large first pass effect and low oral bioavailability. Therefore loads of the drug is cleared in the liver before reaching the target tissues. Therefore it would be better to not give this drug orally
|
|
|
What is oral bioavalibility? |
Normal dose- extraction ratio |
|
|
What should you do differently when prescribing drugs for patients with hepatic impairment? |
-use drugs eliminated by routes other than the liver
-avoid hepatotoxic drugs, sedatives, diuretics, -avoid drugs which cause constipation as these precipitate encephalopathy -avoid drugs that interfere with haemostasis e.g. aspirin, warfarin -avoid drugs that cause sodium retention e.g. NSAIDs, steroids or sodium-containing drugs such as antacids, which exacerbate fluid overload and ascites |
|
|
What lifestyle interventions can help with the management of dyspepsia? |
stop smoking, reduce alcohol, lose weight, increase exercise, reduce fatty or spicy foods, eat small regular meals, raise head of bed
|
|
|
What drugs should be removed to help eliviate the symptoms of dyspepsia? |
-Calcium antagonists and theophyllines cause gastritis
-nitrates cause reflux -bisphosphonates cause oesophageal erosion and ulceration |
|
|
How can you differentiate between gastric and duodenal ulcers? |
Gastric ulcer pain is worse with meals, whereas duodenal ulcer pain is relieved by eating
|
|
|
What does the Blatchford score measure? |
severity of upper GI bleed
|
|
|
What investigations can be used to identify the presence of H.Pylori? |
H. pylori tests include faecal antigen test, Carbon 13 urea breath test, serum H. pylori antibody test, rapid urease test of endoscopic biopsy sample (not really used as unpleasant)
|
|
|
How do PPI's work and what ADR's are associated with their use? |
PPI diffuses out of blood and is activated by acid in canaliculus and binds proton pump on parietal cells;can cause electrolyte disturbance (lowmagnesium), microscopic colitis, acute interstitial nephritis, C. difficile (especiallywith antibiotics)s
|
|
|
Antacids? |
Antacids (aluminium hydroxide, magnesium carbonate, magnesium trisiliate) raise the pH of stomach, neutralise stomach acid, and bind to and inactivate pepsin
Avoid in hypophosphatemia; can cause constipation and diarrhoea |
|
|
H2 receptor antagonists
|
(ranitidine) competitively inhibit action of histamine on parietal cell
|
|
|
How is H.Pylori eradicated? |
H. pylori eradicated using triple therapy, usually a PPI, amoxicillin and clarithromycin (or metronidazole) for 7 days; PPI for 2 months, then re-test¬
|
|
|
Bulk-forming laxatives |
(bran, methylcellulose, isphagula husk) increase volume of non-absorbable material in gut, distend colon and stimulate peristaltic movement
-Contraindicated in dysphagia, intestinal obstruction, colonic atony, faecal impaction -May cause flatulence, abdominal distension and obstruction -Useful in poor diet but not opioid use |
|
|
Osmotic laxatives |
Osmotic (lactulose, macrogols) increase water content in bowel via osmosis, distend colon and stimulate peristaltic movement. They are contraindicated in bowel obstruction
-May cause flatulence, cramps and abdominal discomfort -Caution is older adults and renal impairment |
|
|
Stimulant laxatives |
Stimulants(senna, danthron, bisacodyl)increase peristalsis and water and electrolyte secretion bythe mucosa; usually used with opioids but contraindicated in obstruction.
-In the shortterm, they may cause cramps and discomfort -In the longterm, they may damage nerve plexuses, causing deterioration of normal intestinal function |
|
|
Faecal softener |
-Faecal softeners (liquid paraffin, docusate sodium) promote defecation by softening or lubricating stool, but they should not be administered in children under 3.
-Long term use of liquid paraffin can impair absorption of fat soluble vitamins (A, D) -No effect alone; must be used with another laxative |
|
|
How should you manage an upper GI bleed? |
For upper GI bleeds, consider adrenaline injection, clips, argon-plasma coagulation, heat probe, haemospray
Withhold NSAIDs, 80mg omeprazole IV STAT, then 8mg/hour IV for 72 hours High dose oral therapy for 2 months Repeat endoscopy 6-8 weeks following treatment for a gastric ulcer |
|
|
What does a rasied faecal calprotectin indicate?
|
Elevated faecal calprotectin indicates the migration of neutrophils to the intestinal mucosa, which occurs during intestinal inflammation, including inflammation caused by inflammatory bowel disease
|
|
|
What does a raised faecal elastase indicate? |
Measurement of stool Elastase 1 allows the diagnosis or exclusion of pancreatic exocrine insufficiency
|
|
|
Loperamise |
-An opiate GI-specific anti-motility agent which reduces GI smooth muscle tone and peristalsis.
-Indicated- Mild infective diarrhoea, IBS, chronic IBD, diarrhoea, and high output stomas. -Contraindicated in severe infective diarrhoea, dysentery and liver disease (risk of accumulation); also in severe UC or C. diff as increases risk of toxic megacolon. |
|
|
SABA |
-(salbutamol) have an acute bronchodilator effect;
- SE's- Fine tremor, anxiety, headache, muscle cramps, palpitation, hypokalaemia (tachycardia, arrhythmia), peripheral vasodilation, myocardial ischaemia, sleep or behaviour disturbance |
|
|
LABA |
(salmeterol) are chemical analogues of salbutamol with a long lipophilic side chain which anchors the drug to the lipid membrane allowing the active portion of the molecule to remain at the receptor site; slow onset, not for relief of acute attack
|
|
|
Muscarinic antagonists
|
-(ipratropium bromide, tiotropium) competitively antagonise effects of endogenous Ach at M3 receptors, relaxing bronchial smooth muscle and decreasing mucus secretion.
-Used more in COPD or adjunct in severe asthma -Used in patients who cannot have SABA or LABA; IHD, tachycardia |
|
|
Theophylline
|
-causes acute bronchodilation as a competitive, non-selective phosphodiesterase inhibitor, causing an increase in cAMP in smooth muscle cells. This activates PKA, inhibiting TNF-α and leukotriene synthesis to reduce inflammation. They may also inhibit the delayed phase, are usually oral with sustained release, and have a narrow therapeutic index and many drug interactions including antibiotics
-Nausea, diarrhoea, tachycardia, headaches, insomnia, irritability, dizziness, arrhythmias in overdose -clarithromycin and ciprofloxacin inhibit its metabolism |
|
|
Leukotriene antagonists
|
(montelukast) inhibit cysteinyl L1 receptor, and extensively metabolise; the metabolites undergo biliary excretion.
|
|
|
IgE monoclonal antibodies for asthma
|
(omalizumab) bind to IgE, used in severe persistent IgE-mediated asthma requiring continuous or frequent oral corticosteroids (>4 courses per year)
|
|
|
Explain the steps of management for asthma |
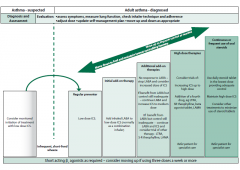
|
|
|
How is acute asthma managed? |
give oxygen, steroids (IV hydrocortisone in severe)
IV magnesium sulphate in severe with peak flow <50% of predicted if not responded to nebulised treatments |
|
|
Describe the management pathway of COPD |
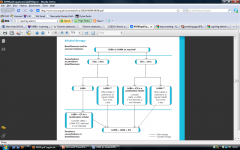
|
|
|
What management options are available for Exacerbation of COPD |
Consider oxygen, short course oral prednisolone, antibiotics (amoxicillin, doxycycline), nebulised bronchodilators and NIV for exacerbations
|
|
|
Define acute severe asthma |
PEF 33-50% of bestor predicted, RR>25, HR>110, orthere is inability to complete sentences in one breathe
|
|
|
Describe life threatening asthma |
PEF<33%, sats <92%, PaO2 <8kPa, normal PaCO2 (getting tired), silent chest, cyanosis, feeble respiratory effort, arrhythmia or hypotension, exhaustion or altered consciousness
|
|
|
Describe the steps of management for HTN |
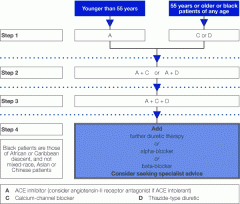
|
|
|
Describe the mechanism of action of Thiazine duiretics |
They control hypertension in part by inhibiting reabsorption of sodium (Na+) and chloride (Cl−) ions from the distal convoluted tubules in the kidneys by blocking the thiazide-sensitive Na+-Cl− symporter.
|
|
|
Describe the ADRs for thiazine diuretics |
May cause postural hypotension (made worse by alcohol), hyponatraemia, hypokalaemia, hypercalcaemia, gout, impaired glucose tolerance, impotence, fatigue, pulmonary oedema, and pneumonitis
Rarely cause thrombocytopenia, photosensitive rash, pancreatitis, renal insufficiency |
|
|
Describe the contraindications for thiazine diuretics |
Avoid in pregnancy as suppresses breast milk production and cause oligohydramnios
Avoid in renal failure, gout and caution in diabetes |
|
|
Describe the mechanism of action of loop diuretics |
Loop diuretics act on the Na+-K+-2Cl− symporter (cotransporter) in the thick ascending limb of the loop of Henle to inhibit sodium, chloride and potassium reabsorption.
|
|
|
What are the ADR's on loop diuretics |
Can cause postural hypotension, tinnitus, photosensitivity, hypokalaemia, renal impairment, gout, urinary retention or frequency
|
|
|
What are the SE's of potassium sparing duiretics |
o May cause electrolyte abnormalities (hyperkalaemia), nausea, vomiting, headache, rash, reduced libido, gynaecomastia
|
|
|
How do Calcium channel blockers work? |
(amlodipine) are particularly effective against large vessel stiffness, a common cause of elevated systolic BP in the elderly. They act on vascular smooth muscle, reducing contraction of arteries and increasing arterial diameter. They also reduce force of contraction of the heart, and slow down electrical activity, slowing the heart rate. They block a calcium signal on the adrenal cortex, reducing aldosterone production
|
|
|
What are the SE's of calcium channel blockers? |
Can worsen proteinuria if patient has nephropathy
Dizziness, headache, redness in face, ankle oedema, rapid or slow heart rate, constipation |
|
|
What are the contraindications of ACE-i's? |
-Caution in impaired renal function, aortic valve stenosis, cardiac outflow obstruction, bilateral renal artery stenosis, hyperkalaemia, hypovolaemia or dehydration
-Contraindicated in pregnancy; congenital malformations and renal problems |
|
|
What are the SE's of ACE-i's? |
May cause hypotension, dry cough, hyperkalaemia, headache, dizziness, fatigue, angioedema, nausea and renal impairment
|
|
|
Describe the mechanism of action of Beta blockers |
competitively antagonise the receptor for endogenous catecholamines epinephrine and norepinephrine at β1 adrenergic receptors, located in the heart and kidneys. They also reduce renin secretion.
|
|
|
What are the SE's of B-blockers |
Nausea, diarrhoea, bronchospasm, cold extremities, exacerbation of Raynaud’s, bradycardia, hypotension, fatigue, dizziness, insomnia, intermittent claudication
|
|
|
What are the contraindications for B-blockers? |
Caution in diabetics, contraindicated in COPD, asthma, heart block
|
|
|
How do NSAID's work? |
NSAIDs block cyclooxygenase and hence prostaglandin synthesis
COX1 has routine physiological functions; COX2 is induced by pain and inflammation Increased cardiovascular risk with COX2 selective but COX1 associated with GI risk Caution prescribing in elderly, renal impairment, liver disease, heart failure |
|
|
How does codeine work? |
Metabolised to morphine by CYP2D6
|
|
|
Describe the how codeine metabolism varies person to person |
Metabolising capacity varies from poor to extensive in population
10% Caucasians poor metabolisers, so have inadequate relief Rapid metabolisers have higher risk of side effects Cirrhosis may prevent metabolism, rendering pain relief ineffective |
|
|
What is tramadol? |
-synthetic opioid with multiple active metabolites
-not completely reversed by naloxone -inhibits serotonin reuptake → mood boost, but may cause serotonin syndrome in combination with SSRI/SNRI -anticholinergic side effects such as urinary retention -contraindicated in severe renal or hepatic failure, raised ICP |
|
|
Give some examples of some common adjunct analgesics |
Corticosteroids, anti-depressants, anticonvulsants, muscle relaxants, bisphosphonates, antispasmodics |
|
|
What are the indications for using corticosteroids as adjunct analgesia? |
Raised ICP, nerve compression, liver capsule pain, soft tissue infiltration |
|
|
What are the indications for using antidepressents/ anticonvulsants as adjunct analgesia? |
Neuropathic pain |
|
|
What are the indications for using muscle relaxants/ bisphosphinates as adjunct analgesia? |
Muscle spasm/ cramp or bone pain |
|
|
What are the indications for using antispasmodics as adjunct analgesia? |
Bowel colic, bladder spasm |
|
|
Give some examples of weak opioids |
codeine, tramadol, dihydrocodeine, co-codamol, co-dydramol
|
|
|
Give some examples of strong opioids |
morphine, diamorphine, hydromorphone, oxycodone, fentanyl
|
|
|
Mophine |
used in acute severe and chronic pain, acts on µ-opioid receptors in CNS
euphoria, bronchospasm, urinary retention, pruritis, hypotension Consider breakthrough pain (1/6 to 1/10 of total regular dose in 24 hours) |
|
|
Describe how to calculate the doses of strong opiods |
20-30mg in opioid naïve, 40-60mg for patients switched from regular weak opioid.
Immediate release 4 hourly or modified release 12 hourly with rescue doses Breakthrough repeated every 2-4 hours as required Oral to IV/SC is 2:1; morphine to oxycodone is 1.5:1; PO morphine to diamorphine is 3:1 Shouldn’t increase regular dose of morphine by over 50% over 24 hours |
|
|
What are the symptoms of opioid toxicitiy? |
myoclonic jerks, pinpoint pupils, hallucinations, confusion, reduced RR
|
|
|
How should you manage opiod overdose? |
consider naloxone if difficult to rouse, RR<8 and/or saturations <90%
slow titration as not to reverse pain relief reduce opiate dose by 30-50%, check renal function, consider alternative |
|
|
How can opioid side effects be managed? |
-fentanyl or tramadol may be less constipating
-adjunct treatments such as ketamine or lidocaine patches so can reduce dose -excluding other causes; bowel obstruction, hypercalcaemia in bowel cancer |
|
|
What are the main symptoms that we should manage in end of life care? |
pain, excess secretions, agitation, nausea, and breathlessness
|
|
|
What is commonly prescribed for N&V in end of life care? |
Cyclizine or haloperidol |
|
|
What is commonly prescribed for agitation in end of life care? |
Midazolam |
|
|
What is commonly prescribed for resp secretions in end of life care? |
hyoscine hydrobromide or
hyoscine butylbromide |
|
|
Define allodynia |
a painful response to a non-painful stimuli
|
|
|
Define Dysaesthesia |
abnormal unpleasant sensation to touch
|
|
|
What are the main drugs available for neuropathic pain? |
-Gabapentin first line in acute pain, acts on calcium channels, and may cause dizziness and ataxia early on but these usually go away with continued use
-Pregabalin is similar but with a quicker onset than gabapentin -Amitriptyline has central and peripheral action on histamine, muscarinic and serotonergic receptors, but must wait two weeks for full effects |
|
|
Flucloxacillin
|
beta Lactam. Is only active against gram positive bacteria, used in skin infections due to good penetration of soft tissues and action against Staph. aureus
|
|
|
Amoxicillin |
Beta Lactam
gram positive activity also has some gram negative activity, and can cross the blood-brain barrier if the meninges are inflamed |
|
|
Co-amoxiclav |
Bea lactam. has good gram positive, gram negative, and anaerobic activity and good soft tissue penetration, used in intra-abdominal and skin infections
|
|
|
Tazocin
|
Beta lactam Has even better gram negative cover than others and may cause less C. diff |
|
|
Cephalexin |
1st generation cephlasporin has mixed activity with good skin and urine penetration but poor penetration of the chest and abdomen |
|
|
Cefuroxime |
2nd generation cephlasporin can only be used IV, with broader cover but no anaerobic activity |
|
|
Cefotaxime
|
3rd generation cephlasporin only IV and penetrates the blood-brain barrier if the meninges are inflamed; ceftriaxone is another 3rd generation cephalosporin |
|
|
What are the main differences between 1st/2nd/3rd generation cephlasporins |
Higher generations have better gram negative cover, however this is at the expense of a loss in gram positive cover |
|
|
How do macrolides work? |
Inhibit protein synthesis by inhibiting binding at 50S ribosomal subunit
|
|
|
How microbes are covered by macrolide treatment? |
Good penetration to most tissues gram positive, anaerobic and some gram negative cover
|
|
|
How do tetracyslines work? |
Bacteriostatic, inhibit protein synthesis by preventing tRNA binding at 30S ribosomal subunit
|
|
|
What SE's and contraindications are associated with tetracyline use? |
Contraindicated in children, pregnancy and breast feeding as deposited in teeth and bones causing discolouration and hyperplasia
GI disturbance, hepatotoxicity, photosensitivity |
|
|
How are aminoglycosides given? |
IV or topical, renal excretion, with concentration-dependent killing; more bacteria killed at higher peak concentrations; so one daily dose, calculated by body weight
|
|
|
What SE's are associated with aminoglycosides? |
Nephrotoxic, ototoxic (augmented by furosemide), precipitates myasthenia gravis
|
|
|
Metronidazole
|
useful for anaerobes and protozoa, forming oxygen free radicals after activation inside the bacteria which interact with nucleic acid
-IV, oral, and rectal (IBD), penetrate BBB and abscesses -hepatic metabolism and renal excretion -disulfiram reaction with alcohol; ‘worst hangover ever’ very soon after drinking |
|
|
Trimethoprim
|
-acts on folate metabolism (dihydrofolate reductase), inhibiting DNA synthesis
-Combined with sulphamethoxazole (co-trimoxazole) -Good gram positive and gram negative activity, frequently used in UTI -Caution renal failure, pregnancy (neural tube defects), can suppress bone marrow (avoid with methotrexate) |
|
|
Nitrofurantoin
|
bactericidal by an unknownmechanism, with renal excretion; active concentration only in urine
|
|
|
What are the common causes of cellulitis |
Staph Aureus The second most common cause is β-haemolytic Strep |
|
|
What is the common cause of infective endocarditis in the prosthetic valve? What Abx would you use? |
In prosthetic valve, Staph. aureus is most common
flucloxacillin (vancomycin, gentamicin if penicillin allergic) and rifampicin |
|
|
What is the common cause of infective endocarditis in the native valve? What Abx would you use?
|
In native valve, caused by Strep. viridans and other α-haemolytic Strep
Benzylpenicillin and gentamicin |
|
|
What antibiotics are used in MRSA? |
Use vancomycin and gentamicin for MRSA
|
|
|
What are the main management options in sedentary patients with AF? |
-beta blocker – atenolol, bisoprolol
-don’t use amiodarone for rate control in persistent AF -CCBs (non-dihydropyridine – diltiazem, verapamil) for L-type calcium channel inhibition; cause negative inotropy and chronotropy. |
|
|
What drugs are available for pharmacological cardioversion? |
Pharmacological cardioversion is with a class III agent (amiodarone, need an IV loading dose) or a class Ic agent (flecanide, propafenone)
|
|
|
Class Ic anti-arrhythmics |
more effective at sinus rhythm restoration than amiodarone if given early (within 12 hours of onset); by 24 hours, there is no difference. They also increase risk of fatal ventricular arrhythmia in those with structural heart disease. Flecainide most appropriate in younger patient
|
|
|
Amiodarone
|
less likely to cause heart failure and hypertension through suppressing myocardial contractility; given in those with structural heart disease
|
|
|
Other than for cardioversion itself when is amioderone indicated? |
6 weeks before and up to a year after cardioversion
|
|
|
What are the main CYP450 enzyme inducers? |
Alcohol, rifampicin, St John’s Wort, carbamazepine, and phenytoin are warfarin enzyme inducers
|
|
|
What are the main CYP450 enzyme inhibitors? |
Amiodarone, simvastatin, macrolides, ciprofloxacin, tramadol and grapefruit juic
|
|
|
What drugs can be specifically tested for in poisoning? |
salicylate, carboxyHb (CO), lithium, paraquat, iron, methanol
|
|
|
What dose of activated charcoal should be used? |
give 10x dose of poison, up to 50g
|
|
|
What risks are associated with the use of activated charcoal? |
Can risk aspiration pneumonia, reduced absorption of therapeutic agents, bowel obstruction
|
|
|
What drugs are not affected by activated charcoal? |
Ineffective for lithium, iron, boron salts, cyanide, glycol, methanol, hydrocarbons, insecticides and strong acids or alkalis
|
|
|
When is Haemodialysis or haemoperfusion used in poisoning? |
may be useful when the poison has a small volume of distribution, low inherent clearance rate, and
is small enough to cross dialysis machine for haemodialysis is bound to activated charcoal for haemoperfusion |
|
|
What are poor prognostic indicators in paracetamol overdose |
PT or INR rising after day 3, PT>180s, bilirubin>70, metabolic acidosis, encephalopathy, raised lactate and creatinine>300
|
|
|
What is the main side effect associated with N-acetyl cysteine treatment? |
Anaphylactoid reaction with urticarial rash, wheeze, hypotension
Not immune-mediated, drug reaction due to dose-related histamine release. reduce infusion rate and give antihistamines, steroids not indicated |
|
|
What are the symptoms of aspirin overdose |
Dizziness, sweating, tinnitus, vomiting, vasodilation, hyperventilation, agitation, delirium, coma, hypoglycaemia, hypokalaemia
|
|
|
How is asprin overdose managed? |
Use activated charcoal, consider MDAC, and prevent CNS penetration by giving sodium bicarbonate to retain drug in its ionised form in the bloodstream.
Enhance elimination by giving bicarbonate; alkalinise the pH of the urine. Haemodialysis highly effective at removing salicylate and also corrects metabolic abnormalities. Consider is pH<7.3, salicylate over 700mg/l, or if the patient is in renal failure. |
|
|
What are the signs and symptoms of TCA overdose? |
Anticholinergic effects including hot dry skin, dilated pupils, tachycardia, urinary retention, agitation, delirium, fits, coma, hypertonia, hyperreflexia. Sodium channel blocking affects cause cardiac arrhythmias (VT), conduction block, prolonged QRS and QT intervals. α-adreno-receptor antagonism causes hypotension
|
|
|
What is the management for TCA overdose? |
ECG is essential- assess QRS duration Charcoal often not used If acidotic give sodium bicarbonate |
|
|
What are the symptoms of Iron overdose? |
Early (<6hours) – nausea, vomiting, abdominal pain, bloody diarrhoea, massive GI fluid loss
Delayed (2-72 hours) - black offensive stools, drowsiness, coma, fits, circulatory collapse, haematemesis, metabolic acidosis, hypotension, positive anion gap Late (2-4 days) - acute liver necrosis and renal failure; very late (2-5 weeks) - gastric strictures |
|
|
What is the treatment for iron poisoning? |
Desferrioxaminechelates iron and reduces toxicity; chelate is water-solubleand excreted in urine with red discolouration
|

