![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
What are the key fuels for the body? |
Stored fuels = glycogen and triglyceride
Circulating as = glucose and NEFAs, ketones and trigylcerides
Metabolised as = glucose, NEFA and ketones |
|
|
What is the main energy supply during fasting? |
Most energy supply comes from lipid
|
|
|
Describe the fasting state: |
Plasma glucose < 5mmolar No glucose is being stored Glucose is mobilised from glycogen by the brain, red blood cells and skin. High plasma fatty acids being oxidised by most other tissues. Some stress hormones e.g. cortisol and sympathetic: increase with prolonged fasting. |
|
|
Describe the fed state: |
Most energy supply is coming from glucose. High insulin Plasma glucose is 7-8mmolar Most glucose is being stored as glycogen Low fatty acids Glucose is being taken up be skeletal muscle and metabolised by the brain, RBCs and skin. Low stress hormones. |
|
|
Describe the state of the blood during exercise: |
Increased fuel needs met by increased use of both lipid and glucose. Low insulin Normal plasma glucose 5 mmolar. No glucose being stored, glucose is being mobilised form glycogen glucose being metabolised by skeletal muscle, brain, RBCs, skin and others. Aerobic and anaerobic. high stress hormones.
|
|
|
Compare and contrast the levels of insulin, glucagon, epinephrine and cortisol during a. fed state, b. fasting state and c. exercise. |
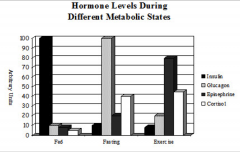
|
|
|
Describe glycolysis, lipolysis, Beta oxidation, ketone body formation and gluconeogenesis: |
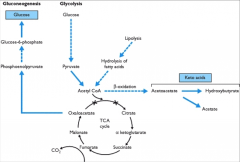
|
|
|
What are the key fuels for: 1. the brain 2. RBCS and Skin 3. Skeletal muscle - resting state, exercise state, fed state - glucose. |
1. glucose, aerobic 2. glucose = anaerobic 3. resting = mostly fatty acids, exercise = mostly fatty acid but glucose oxidation increases too, fed state= glucose.
|
|
|
How much insulin is produced per day? |
About 30 units/ per day. Insulin requirement is increased in obesity and inactive people. Less insulin requirements in active,fit people.
|
|
|
Define diabetes mellitus: |
Always <30% of beta-cell function left. Type 1 usually about 10% when they present, usually <5% in most patients.
In type 1 diabetes = absolute insulin deficiency In type 2 = relative insulin deficiency. - may still be making 40 units per day but need more.
|
|
|
What is the defining feature of diabetes mellitus? |
Blood sugar levels |
|
|
Describe Type 1 diabetes: |
Chronic autoimmune disease with destruction of the beta-cells in the islet of Langerhans in the pancreas. Characterised by immune-T cell-mediated disruption of the insulin secreting B cells within the islets of Langerhans in the pancreas. Genetic component HLA alleles (autoimmune thyroid disease, addison's disease and pernicious anaemia) increases susceptibility 90% possess autoantibodies. Prevalence of 0.1-> 0.2 of caucasian pop. Most common at 7-13 yrs. Can occur at any age. Not preventable currently. Highest rates in FInland and Northern Europe. Increasing incidence in most populations. |
|
|
Differentiate the presentation of type 1 and type 2 diabetes: |
Type 1: younger and more sudden onset, 30% present as an emergency. more weight loss and never have diabetic tissue damage.
|
|
|
How can type 1 diabetes be managed day to day? |
Monitor blood glucose. Replace insulin Eat regularly, timing and quantities. Adapt to changes in exercise, food and other illness/stress.
|
|
|
What are the symptoms of hypoglycaemia? |
Dizziness, visual distubrances, hunger, confusion, personality changes, aggression, coma.
Sympathetic response: sweating, pallor, tremor and nausea. |
|
|
What are the symptoms of high blood sugar? |
SYmptoms above > 10 Mmolar.
-thirsy, polyuria, nocturia, nausea, tired, infections. -renal and eye problems -nerve problems -blood vessel damage. |
|
|
What causes ketoacidosis? |
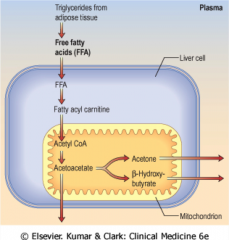
Severe insulin deficiency results in high blood sugar, high NEFA and excess ketone production dehydration, very thirsty and nauseated breathlessness.
TAG --> FFA ---> transported into the liver cell ---> fatty acyl carnitine ---> acetyl coA ---> acetoacetate ---> acetone and Beta hydroxybutyrate. |
|
|
What is the prevelance of diabetes in the UK? |
6% of the population in England.
347 milion people worldwide. 1.5 million deaths directly from diabetes. will be the 7th leading cause of death in 2030. |
|
|
State 3 key biochemical aspects to type 1 diabetes: |
1. Insulin deficiency = need for constant insulin injections 2. May develop ketoacidosis 3. Disappearance of C-peptide. |
|
|
Describe type 2 diabetes: |
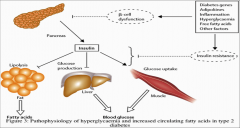
Most common (85-90% of cases) Chronic and progressive disease characterised by abnormal insulin action and secretion. Result is increased lipolysis, increased glucose production and decreased glucose uptake.
Most commonly in older patients but high prevalence in overweight and obese. NO HLA links 50% concordance in identical twins RIsk to non-identical twins or siblings is 25%. Genetic component to the disease but the genes responsible for most of the cases are unknown. Environmental infleunces: BMI greater than 31 leads to a 40X increased risk. 1st generation family histroy, predispositon even lean healthy individuals develop skeletal muscle insulin deficiency. Age: increased mitochondrial dysfunction with age plus inflammation. |
|
|
What is insulin resistance? |
Often present for years before diabetes is diagnosed, reflects a diminished response to insulin and its key targets. |
|
|
What are the causes of insulin resistance? |
1. Accumulation of lipids and their metabolites or increased concentration of circulating free fatty acids. 2. Circulating cytokines released by infiltration of macrophages into adipose tissue. 3. Obesity: increased lipid levels, chronic inflammation and altered adipokine levels. 4. Hyperinsulinaemia: increases lipid synthesis and exacerbates insulin resistance. |
|
|
Describe the mechanisms of insulin resistance: |
Insulin binds to its receptor on the plasma membrane. This causes phosporylation of IRS which binds to the regulatory subunit of P13K
PI3K converts PIP2 to PIP3.
PIP3 activated PDK1 which activates Akt and causes insulin mediated intracellular functions. |
|
|
What signalling is involed in insulin resistnace? |
THEORIES: -DAG induced activation of PKC isoforms.
-Pro-inflammatory cytokines mediated activation of stress kinase c-Jun N-terminal kinase.
- ER stress
|
|
|
In helathy individuals what is insulin resistance associated with? |
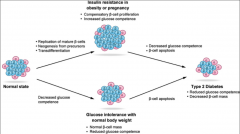
Physiological conditions such as pregnancy or body weight gain. New beta cells can be generated in response to insulin resistance associated with obesity or pregnancy. |
|
|
What islet compensation occurs in insulin resistance? |
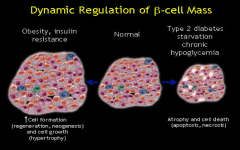
Normally islets comprise 1-2% of the pancreatic cell population and B cells represent 60% of the islet endocrine cells. In insulin resistance islets increase in both size and number due to Beta cell increases in size and number with the Beta cell constituting up to 90% of the cells.
|
|
|
What happens to the islet cells in T.2 Diabetes? |
Significant reduction in the number of beta cells per islet.
Atrophy and cell death of the beta cells. reduced pancreatic B cell mass and increased death with B cell dysfunction.
|
|
|
What is the most important way in which diabetes can develop? |
Impaired insulin secretion rather than insulin action.
Inherited abnormalities of B cell function of mass or both are critical precursors in type 2 diabetes. |
|
|
State some the causes of pancreatic B cell loss of function |
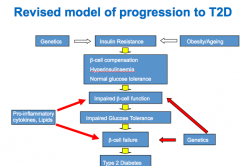
Reduced B cell mass Reduced insulin synthesis Reduced insulin secretion
B cell failure due to: glucose toxicity, ^ FFA, ^ TG, TNF alpha, age increasing, genetics. |
|
|
State 3 other forms of DM: |
1. Maturity onset diabetes of the young (AD) present at birth often detected later in life.
2. Gestational diabetes. occurs in 2-6% of pregancies in European women. Increases risk of subsequent T.2 DM.
3. Latent Autoimmune diabetes of adults (type 1.5 diabetes) |
|
|
How is diabetes diagnosed? |
Diagnosed on the basis of one abnormal plasma glucose. random >= 11.1 mmol/L or fasting >= 7 mmol/L in the prescence of diabetes symptoms of thirst, increaesed urination, recurrent infections, wieght loss, drowsiness and coma.
In asymptomatic people with an abnormal random plasma glucose, 2 fasting venous plasma glucose samples (>+ 7 mmol/L are recommended). |
|
|
Explain the use of an oral glucose tolerance test is diagnosis of diabetes: |
Overnight fast Base-line blood glucose measured. glucose drink is consumed 75g glucose blood glucose measured 2 hrs after.
DM: Fasting: plasma glucose > 7.0 2hrs: > 11.1
|
|
|
Diagnosis using HbA1c |
Level of HbA1c >= 6.5 for diagnosis.
Advantages: reliable measure: HbA1C levels are relatively stable. ease of sampling. patient convenience no need for overnight fast.
Limitations: cost in some countries. influence of Hb disorders HbS, HbF conditions change turnover.
|
|
|
What are the 2 aims of T2D treatment? |
Treat: with the aim of lowering blood glucose levels.
1. Insulin resistance
2. Beta cell dysfunction
|
|
|
State the key drugs given: |
1. Metformin (inhibitd gluconeogenesis)
2. Sulfonylureas: Activate the ATP-sensitive K+ channels.
3. DPP-4 inhibitors: block DPP-4 and therefore increases GLP-1 which stimulates insulin release and inhibits glucagon release. |
|
|
What is the normal mechanism of insulin secretion? |
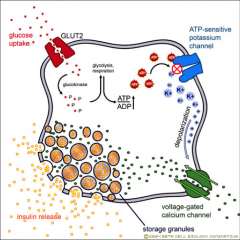
Glucose uptake into cells by GLUT 1 Glucose is metabolised and this increases ATP: ADP ratio. This activates ATP-senstive K+ channels with CLOSE and depolarise the cell. Depolarisation opens the Ca2+ channels and this influx of Ca2+ causes the translocation of insulin vesicles to the membrane surface resulting in insulin release. |
|
|
What are the acute complications of diabetes? |
Aucte: reduced insulin production of action --> Ketoacidosis (continual use of Fas for energy leads to the production of ketone bodies: acetoacetate and Beta-hydroxybutrate)
Blood and urine acid levels rise, dehydration, coma and death.
Potentially life-threatening complications more common in T1DM but posibile
Hypoglycaemia |
|
|
What are the chronic complications of diabetes? |
Hyperglycaemia: macrovascular: atherosclerosis: CVD
Microvascular: kidney disease (nephropathy nerve disease (neuropathy) Blindness (retinopathy) Amputation
Dyslipidaemia : ectopic fat deposition in skeletal muscle and liver exacerbation of insulin resistance, macrovascular complications. |
|
|
What is the Polyol pathway? |
Sorbitol accumulates and promotes osmotic stress and cell damage: Diabetic retinopathy.
Decreases ATP- Sodium/Potassium pump in neurones leading to nerve damage = diabetic neuropathy.
Decreases in NO levels leading to blood vessel constriction and hypertension and microvascular and macrovascular damage.
Increases free radical production cellular damage and contributes to micro and macrovasc damage. |
|
|
What is the effevt of protein Kinase C activation? |
Increased reactive oxygen species levels. Increased inflammation Mitochondrial dysfunction
|
|
|
Describe the 3 major microvascular complications of diabetes: |
Diabetes retinopathy: occurs in most people after 20 years of poorly controlled diabetes. diabetes is the leading cause of new blindness or partial vision loss in people aged 20-74. damage to the blood vessels in and around the eye.
Diabetic nephropathy: characterised by proteinuria, glomerular hypertrohpy, decrease GFR and renal fibrosis with loss of renal function. the leading cause of end-stage renal disease. 1/3rd of diabetes patients are affected.
Diabetic neuropathy: 60-70% of people with diabetes develop some form of nerve disease. Nerve fibres and blood vessels supplying nerves are damaged.
|
|
|
What are the key macrovascular complications of diabetes? |
= metabolic injury to the large blood vessels =arteries and veins = atherosclerosis. most common cause of death in diabetes (70% of deaths) --Tissue nutrient and oxygen supply to the heart, brain and extremities is compromised. --Cerebrovascular disease (stroke) --MI, congesitve HF |

