![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
368 Cards in this Set
- Front
- Back
|
CHAPTER 6: Cardiovascular/Renal Agents
|
CHAPTER 6: Cardiovascular/Renal Agents
|
|
|
ANTIARRHYTHMIC (ANTIDYSRRHYTHMIC) AGENTS
|
ANTIARRHYTHMIC (ANTIDYSRRHYTHMIC) AGENTS
|
|
|
Describe what happens during each of the following phases of the cardiac action potential for fast-response fibers:
Phase 0 |
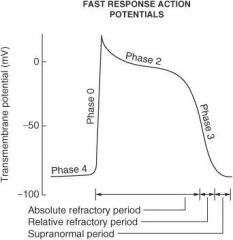
Sodium ion channels open (inward) which leads to membrane depolarization.
|
|
|
Describe what happens during each of the following phases of the cardiac action potential for fast-response fibers:
Phase I |
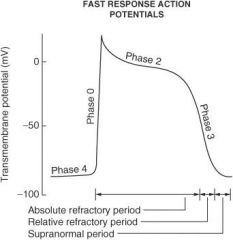
Sodium ion channels are inactivated; potassium ion channels (outward) are activated; chloride ion channels (inward) are activated.
|
|
|
Describe what happens during each of the following phases of the cardiac action potential for fast-response fibers:
Phase II |
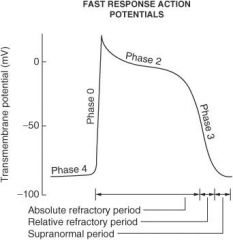
Plateau phase; slow influx of calcium ion balanced by outward potassium ion current (delayed rectifier current IK)
|
|
|
Describe what happens during each of the following phases of the cardiac action potential for fast-response fibers:
Phase III |
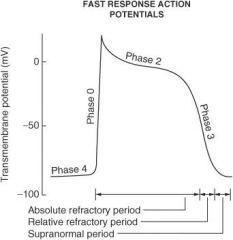
Repolarization phase; outward K⁺ current increases and inward calcium ion current decreases
|
|
|
Describe what happens during each of the following phases of the cardiac action potential for fast-response fibers:
Phase IV |
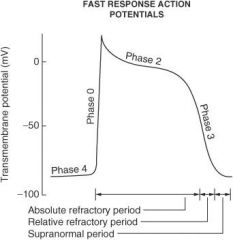
Membrane returns to resting potential.
|
|
|
On what phase(s) of the cardiac action potential do amiodarone and sotalol work?
|
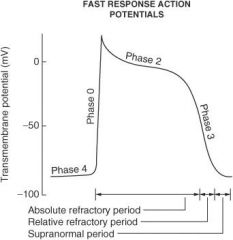
Phase 0 and phase III
|
|
|
On what phase(s) of the cardiac action potential do lidocaine, flecainide, and quinidine work?
|
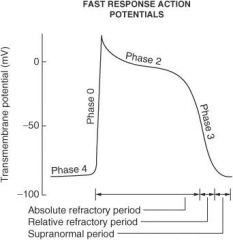
Phase 0
|
|
|
On what phase(s) of the cardiac action potential do β-blockers work?
|
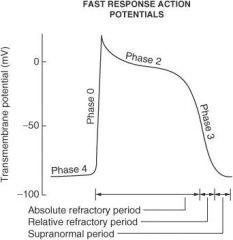
Phase II and phase IV
|
|
|
What is responsible for maintaining the electrochemical gradient at resting membrane potential?
|
Na⁺/K⁺-ATPase
|
|
|
What ion current is responsible for the depolarization of sinoatrial (SA) and atrioventricular (AV) nodal fibers?
|
Calcium ion (inward)
|
|
|
What ion current is responsible for the repolarization of SA and AV nodal fibers?
|
Potassium ion (outward)
|
|
|
How does phase IV of the action potential in slow-response fibers (SA and AV nodes) differ from that of fast-response fibers?
|
Slow-response fibers display automaticity (ability to depolarize spontaneously); rising phase IV slope of the action potential = pacemaker potential
|
|
|
What ion current is responsible for the 'pacemaker current' (rising slope of phase IV) in slow-response fibers?
|
Sodium ion (inward); calcium ion (inward); potassium ion (outward)
|
|
|
The pacemaker of the heart has the fastest uprising phase IV slope; where is this pacemaker in nondiseased patients?
|
SA node
|
|
|
Where is the SA node located?
|
Right atrium
|
|
|
How do the effective refractory period (ERP) and relative refractory period (RRP) differ from each other?
|
No stimulus, no matter the strength, can elicit a response with fibers in the ERP, whereas a strong enough stimulus will elicit a response with fibers in the RRP.
|
|
|
What are the three states the voltage-gated Na⁺ channel exists in?
|
① Resting state
② Open state ③ Inactivated state |
|
|
What state(s) of the voltage-gated Na⁺ channel is/are most susceptible to drugs?
|
Open state; inactivated state
|
|
|
What two types of gates does the voltage-gated Na⁺ channel have?
|
① M (activating)
② H (inactivating) |
|
|
Why is the rate of recovery from an action potential slower in ischemic tissue?
|
The cells are already partly depolarized at rest.
|
|
|
What class of antiarrhythmic agents has membrane-stabilizing effects?
|
β-Blockers
|
|
|
Antiarrhythmic agents are grouped into four classes according to what classification system?
|
Vaughn-Williams classification
|
|
|
Give the general mechanism of action for each of the following antiarrhythmic drug classes:
Class I |
Na⁺ channel blockers
|
|
|
Give the general mechanism of action for each of the following antiarrhythmic drug classes:
Class II |
β-Blockers
|
|
|
Give the general mechanism of action for each of the following antiarrhythmic drug classes:
Class III |
K⁺ channel blockers
|
|
|
Give the general mechanism of action for each of the following antiarrhythmic drug classes:
Class IV |
Ca²⁺ channel blockers
|
|
|
Class I antiarrhythmics are further subdivided into what classes?
|
la; Ib; Ic
|
|
|
Give examples of antiarrhythmic drugs in class la:
|
Quinidine (antimalarial/antiprotozoal agent); procainamide; disopyramide
|
|
|
Give examples of antiarrhythmic drugs in class Ib:
|
• Lidocaine
• mexiletine • tocainide • phenytoin |
|
|
Give examples of antiarrhythmic drugs in class Ic:
|
• Encainide
• flecainide • propafenone • moricizine |
|
|
Give examples of antiarrhythmic drugs in class II:
|
• Propranolol
• esmolol • metoprolol |
|
|
Give examples of antiarrhythmic drugs in class III:
|
• Amiodarone
• sotalol • ibutilide • dofetilide |
|
|
Give examples of antiarrhythmic drugs in class IV:
|
Verapamil; diltiazem
|
|
|
Name three antiarrhythmic drugs that do not fit in the Vaughn-Williams classification system:
|
① Digoxin
② Adenosine ③ Magnesium |
|
|
Magnesium is used to treat what specific type of arrhythmia?
|
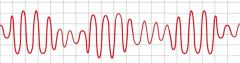
Torsades de pointes (polymorphic ventricular tachycardia)
|
|
|
Adenosine is used to treat what types of arrhythmias?
|
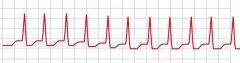
Paroxysmal supraventricular tachycardia (PSVT - shown), specifically narrow complex tachycardia or supraventricular tachycardia (SVT) with aberrancy; AV nodal arrhythmias (adenosine causes transient AV block). Note: synchronized cardioversion and not adenosine should be used on symptomatic patients or unstable tachycardia with pulses.
|
|
|
Where anatomically should the IV be placed to administer adenosine?
|
As close to the heart as possible, that is, the antecubital fossa since adenosine has an extremely short half-life. Adenosine rapid IV push should be followed immediately by a 5-10 cc (mL) flush of saline to facilitate its delivery to the heart.
|
|
|
What is the mechanism of action of adenosine?
|
Stimulates α₁-receptors which causes a decrease in cyclic adenosine monophosphate (cAMP) (via Gs coupled second messenger system)
increases K⁺ efflux leading to increased hyperpolarization; increases refractory period in AV node |
|
|
What are the adverse effects of adenosine?
|
• Flushing
• chest pain • dyspnea • hypotension |
|
|
What two drugs can antagonize the effects of adenosine?
|
① Theophylline
② Caffeine |
|
|
How is adenosine dosed?
|
6 mg initially by rapid IV push; if not effective within 1-2 minutes, give 12 mg repeat dose (follow each bolus of adenosine with normal saline flush). The 12 mg dose may be repeated once.
|
|
|
What is the most deadly ion that can be administered?
|
Potassium ion
|
|
|
What ECG changes are seen in hyperkalemia?
|
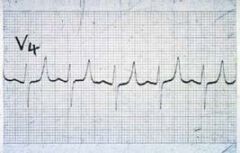
• Flattened P waves
• widened QRS complex • peaked T waves • sine waves • ventricular fibrillation |
|
|
What ECG changes are seen in hypokalemia?
|
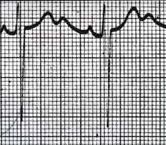
Flattened or inverted T waves; U waves; ST-segment depression
|
|
|
What do class la antiarrhythmics do to each of the following?
Action potential duration |
Increase
|
|
|
What do class la antiarrhythmics do to each of the following?
ERP |
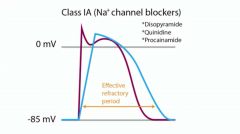
Increase
|
|
|
What do class la antiarrhythmics do to each of the following?
Conduction velocity |
Decrease
|
|
|
What do class la antiarrhythmics do to each of the following?
Phase IV slope |
Decrease
|
|
|
What do class Ib antiarrhythmics do to each of the following?
Action potential duration |
Decrease
|
|
|
What do class Ib antiarrhythmics do to each of the following?
ERP |

Little or no change
|
|
|
What do class Ib antiarrhythmics do to each of the following?
Conduction velocity |
Decrease (primarily in ischemic tissue)
|
|
|
What do class Ib antiarrhythmics do to each of the following?
Phase IV slope |
Decrease
|
|
|
What do class Ic antiarrhythmics do to each of the following?
Action potential duration |
Little or no change
|
|
|
What do class Ic antiarrhythmics do to each of the following?
ERP |

Little or no change
|
|
|
What do class Ic antiarrhythmics do to each of the following?
Conduction velocity |
Decrease
|
|
|
What do class Ic antiarrhythmics do to each of the following?
Phase IV slope |
Decrease
|
|
|
Drugs that affect the strength of heart muscle contraction are referred to as what types of agents?
|
Inotropes (either positive or negative)
|
|
|
Drugs that affect the heart rate are referred to as what types of agents?
|
Chronotropes (either positive or negative)
|
|
|
Drugs that affect AV conduction velocity are referred to as what types of agents?
|
Dromotropes (either positive or negative)
|
|
|
QT interval prolongation, and therefore torsades de pointes, is more likely to occur with what two classes of antiarrhythmics?
|
① la
② Ill |
|
|
Which class la antiarrhythmic also blocks α-adrenergic and muscarinic receptors, thereby potentially leading to increased heart rate and AV conduction?
|
Quinidine
|
|
|
What are the adverse effects of quinidine?
|
• Tachycardia
• proarrhythmic • increased digoxin levels via protein-binding displacement • nausea & vomiting • diarrhea • cinchonism |
|
|
What is cinchonism?
|
• Syndrome that may include tinnitus
• high-frequency hearing loss • deafness • vertigo • blurred vision • diplopia • photophobia • headache • confusion • delirium |
|
|
What are the adverse effects of procainamide?
|
• Drug-induced lupus (25%-30% of patients)
• proarrhythmic • depression • psychosis • hallucination • nausea & vomiting • diarrhea • agranulocytosis • thrombocytopenia • hypotension |
|
|
What drugs can cause drug-induced lupus?
|
• Procainamide
• isoniazid (INH) • chlorpromazine • penicillamine • sulfasalazine • hydralazine • methyldopa • quinidine • phenytoin • minocycline • valproic acid • carbamazepine • chlorpromazine |
|
|
Which class la antiarrhythmic can cause peripheral vasoconstriction?
|
Disopyramide
|
|
|
What are the adverse effects of disopyramide?
|
• Anticholinergic adverse effects, such as urinary retention
• dry mouth • dry eyes • blurred vision • constipation • sedation |
|
True or False?
Lidocaine is useful in the treatment of ventricular arrhythmias? |
TRUE
|
|
True or False?
Lidocaine is useful in the treatment of atrial arrhythmias? |
FALSE
|
|
True or False?
Lidocaine is useful in the treatment of AV junctional arrhythmias? |
FALSE
|
|
|
What are the adverse effects of lidocaine?
|
• Proarrhythmic
• sedation • agitation • confusion • paresthesias • seizures |
|
|
What class Ib antiarrhythmic is structurally related to lidocaine?
|
Mexiletine
|
|
|
What class Ib antiarrhythmic can cause pulmonary fibrosis?
|
Tocainide
|
|
|
Propafenone, even though a class Ic antiarrhythmic, exhibits what other type of antiarrhythmic activity?
|
β-Adrenergic receptor blockade
|
|
|
What famous trial showed that encainide and flecainide increased sudden cardiac death in postmyocardial infarction (MI) patients with arrhythmias?
|
Cardiac Arrhythmia Suppression Trial (CAST)
|
|
|
Sotalol, even though a class III antiarrhythmic, exhibits what other type of antiarrhythmic activity?
|
β-Adrenergic receptor blockade
|
|
|
Even though this agent is labeled as a Vaughn-Williams class III antiarrhythmic, it displays class I, II, III, and IV antiarrhythmic activity.
|
Amiodarone
|
|
|
What is the half-life of amiodarone?
|
40 to 60 days
|
|
|
What are the adverse effects of amiodarone?
|
• Pulmonary fibrosis
• tremor • ataxia • dizziness • hyperthyroidism, hypothyroidism • hepatotoxicity • photosensitivity • blue skin discoloration • neuropathy • muscle weakness • proarrhythmic • corneal deposits • lipid abnormalities • hypotension • nausea & vomiting • congestive heart failure (CHF) • optic neuritis • pneumonitis • abnormal taste • abnormal smell • syndrome of inappropriate secretion of antidiuretic hormone (SIADH) |
|
|
How should patients on amiodarone therapy be monitored?
|
• ECG
• thyroid function tests (TFTs) • pulmonary function tests (PFTs) • liver function tests (LFTs) • electrolytes • ophthalmology examinations |
|
|
Verapamil should not be given in what types of arrhythmias?
|
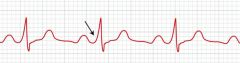
Wolff-Parkinson-White (WPW) syndrome; ventricular tachycardia
|
|
|
What are the adverse effects of verapamil?
|
• Drug interactions
• constipation • hypotension • AV block • CHF • dizziness • flushing |
|
|
Digoxin is used to control ventricular rate in what types of arrhythmias?
|
Atrial fibrillation; atrial flutter
|
|
|
Digoxin-induced arrhythmias are treated by what drugs?
|
Lidocaine; phenytoin
|
|
|
Digoxin does what to each of the following?
Strength of heart muscle contraction |
Increases (positive inotrope)
|
|
|
Digoxin does what to each of the following?
Heart rate |
Decreases (negative chronotrope)
|
|
|
Digoxin does what to each of the following?
AV conduction velocity |
Decreases (negative dromotrope)
|
|
|
What does QTc stand for?
|
Corrected QT interval
|
|
|
How is QTc calculated?
|
(QT)/(square root of R to R interval)
|
|
|
Why must the QT interval be corrected?
|
The QT interval is dependent on heart rate, so higher heart rates will display shorter QT intervals on ECG. It is corrected to remove the variable of the heart rate.
|
|
|
What is the normal value for QTc?
|
Less than 440 milliseconds
|
|
|
What does a long QT interval put a patient at risk for?
|
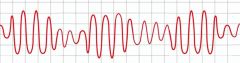
Torsades de pointes, a ventricular arrhythmia that can degenerate into ventricular fibrillation
|
|
|
CONGESTIVE HEART FAILURE AGENTS
|
CONGESTIVE HEART FAILURE AGENTS
|
|
|
What is the cardiac output equation?
|
Cardiac output (CO) = heart rate (HR) × stroke volume (SV)
|
|
|
What is normal CO?
|
5 L/min
|
|
|
What is the most common cause of right-sided heart failure?
|
Left-sided heart failure
|
|
|
Name three compensatory physiologic responses seen in congestive heart failure (CHF):
|
① Fluid retention
② Increased sympathetic drive ③ Hypertrophy of cardiac muscle |
|
|
Define preload:
|
The pressure stretching the ventricular walls at the onset of ventricular contraction; related to left ventricular end-diastolic volume/pressure
|
|
|
Define afterload:
|
The load or force developed by the ventricle during systole
|
|
|
What drugs are used to decrease preload?
|
• Diuretics
• vasodilators • angiotensin-converting enzyme inhibitors (ACEIs) • angiotensin II receptor blockers (ARBs) • nitrates |
|
|
What drugs are used to decrease afterload?
|
• Vasodilators
• ACEIs • ARBs • hydralazine |
|
|
What drugs are used to increase contractility?
|
Digoxin; phosphodiesterase inhibitors (amrinone and milrinone); β-adrenoceptor agonists
|
|
|
What is the mechanism of action of digoxin?
|
Inhibition of the Na⁺/K⁺-ATPase pump which leads to positive inotropic action (via increased intracellular sodium ions that exchanges with extracellular calcium ions; resulting increase in intracellular calcium ions leads to increased force of contraction)
|
|
|
What are the two digitalis glycosides?
|
① Digoxin
② Digitoxin |
|
|
What are the adverse effects of digoxin?
|
• Arrhythmias
• nausea & vomiting • anorexia • headache • confusion • blurred vision • visual disturbances, such as yellow halos around light sources |
|
|
What electrolyte disturbances predispose to digoxin toxicity?
|
Hypokalemia; hypomagnesemia; hypercalcemia
|
|
|
Digoxin can cause what types of arrhythmias?
|
• Supraventricular tachycardias
• AV nodal tachycardias • AV block • ventricular tachycardias • ventricular fibrillation • complete heart block |
|
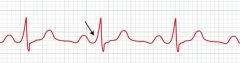
Can digoxin be used in WPW syndrome?
|
No. Since digoxin slows conduction through the AV node, the accessory pathway present in WPW is left unopposed, leading to supraventricular tachycardias and atrial arrhythmias.
|
|
|
How is digoxin toxicity treated?
|
Correction of electrolyte disturbances; antiarrhythmics; anti-digoxin Fab antibody (Digibind)
|
|
|
What drugs can increase digoxin concentrations?
|
• Quinidine
• amiodarone • erythromycin • verapamil |
|
|
What drugs can decrease digoxin concentrations?
|
Loop diuretics; thiazide diuretics; corticosteroids
|
|
|
Does digoxin therapy in CHF lead to prolonged survival?
|
No. It is of symptomatic benefit only, improving quality, but not necessarily duration of life.
|
|
|
What classes of medications have been shown to increase survival in CHF patients?
|
ACEs/ARBs; β-blockers
|
|
|
How does dobutamine work in CHF?
|
β-Adrenergic agonist (sympathomimetic that binds to (β₁-adrenoceptors) that increases force of contraction and vasodilation via increased cAMP
|
|
|
How do amrinone and milrinone work in CHF?
|
• Inhibits phosphodiesterase (PDE) thereby increasing cAMP levels
• increased cAMP leads to increased intracellular calcium • increased intracellular calcium leads to increased force of contraction • increased cAMP also leads to increased vasodilation |
|
|
What are the side effects of the PDEIs?
|
Milrinone may actually decrease survival in CHF; amrinone may cause thrombocytopenia.
|
|
|
How do diuretics work in CHF?
|
Decrease in intravascular volume thereby decrease in preload; reduce pulmonary and peripheral edema often seen in CHF patients
|
|
|
How can increased sympathetic activity in CHF be counteracted?
|
β-Blockers
|
|
|
What two β-blockers have specific indications for the treatment of CHF?
|
① Metoprolol
② Carvedilol (mixed α-/β-blocker) |
|
|
What is the mechanism of action of nesiritide?
|
Recombinant B-type natriuretic peptide that binds to guanylate cyclase receptors on vascular smooth muscle and endothelial cells, thereby increasing cyclic guanosine monophosphate (cGMP) levels; increased cGMP leads to increased relaxation of vascular smooth muscle
|
|
|
How do ACEIs work in CHF?
|
Inhibition of angiotensin-II (AT-II) production thereby decreasing total peripheral resistance (TPR) and thus afterload; prevents left ventricular remodeling
|
|
|
ANTIANGINAL AGENTS
|
ANTIANGINAL AGENTS
|
|
|
Define angina pectoris:
|
Chest pain resulting from a myocardial oxygen demand that is not met by adequate oxygen supply; seen in patient with myocardial ischemia
|
|
|
What type of angina is caused by spontaneous coronary vasospasm?
|
Prinzmetal (variant) angina
|
|
|
What type of angina is caused by atherosclerosis of coronary vessels and is precipitated by exertion?
|
Classic angina
|
|
|
What type of angina can be acute in onset and is caused by platelet aggregation?
|
Unstable angina
|
|
|
What two mechanistic strategies are used in the treatment of angina?
|
① Increase oxygen supply to the myocardium
② Decrease myocardial oxygen demand |
|
|
What types of drugs can increase oxygen supply?
|
Nitrates; calcium channel blockers (CCBs)
|
|
|
What types of drugs can decrease oxygen demand?
|
Nitrates; CCBs; β-blockers
|
|
|
What is the drug of choice for immediate relief of anginal symptoms?
|
Sublingual nitroglycerin (NTG)
|
|
|
What is the mechanism of action of nitrates?
|
• Nitrates form nitrites
• nitrites form nitric oxide (NO) • NO activates guanylyl cyclase to increase cGMP • increased cGMP leads to increased relaxation of vascular smooth muscle |
|
|
How does cGMP lead to relaxation of vascular smooth muscle?
|
Causes dephosphorylation of myosin light chains
|
|
|
How do nitrates increase oxygen supply?
|
Dilation of coronary vessels which leads to increased blood supply
|
|
|
How do nitrates decrease oxygen demand?
|
Dilation of large veins which leads to preload reduction; decreased preload reduces the amount of work done by the heart; decreased amount of work results in decreased myocardial oxygen requirement
|
|
|
What are the adverse effects of nitrates?
|
• Headache
• hypotension • reflex tachycardia • facial flushing • metnemoglobinemia |
|
|
Why must patients have at least a 10- to 12-hour 'nitrate-free' interval every day?
|
Tolerance (tachyphylaxis) develops to nitrates if given on a continuous (around-the-clock) basis
|
|
|
Nitrates are contraindicated in patients taking any of what three medications?
|
① Sildenafil
② Vardenafil ③ Tadalafil |
|
|
Methemoglobin formation, specifically by amyl nitrite, can be used to treat what type of poisoning?
|
Cyanide
|
|
|
What are the common formulations of nitrates?
|
NTG; isosorbide mononitrate; isosorbide dinitrate
|
|
|
What is the time to peak effect of sublingual NTG?
|
2 minutes
|
|
|
What is the dosing frequency of sublingual NTG during an anginal episode?
|
Every 5 minutes for a maximum of three doses
|
|
|
How do β-blockers work in the treatment of angina?
|
Inhibition of α₁-adrenoceptors which leads to decreased CO, HR, and force of contraction, thereby reducing the workload of the heart and oxygen demand
|
|
|
Do α-blockers increase oxygen supply?
|
No
|
|
|
For each of the following CCBs, state whether their primary effects are on the myocardium or peripheral vasculature:
Verapamil |
Myocardium (greater negative inotropic effects)
|
|
|
For each of the following CCBs, state whether their primary effects are on the myocardium or peripheral vasculature:
Dihydropyridines (DHP; nifedipine, amlodipine, felodipine, isradipine, nicardipine) |
Peripheral vasculature (more potent vasodilators)
|
|
|
For each of the following CCBs, state whether their primary effects are on the myocardium or peripheral vasculature:
Diltiazem |
Myocardium
|
|
|
How do CCBs work in the treatment of angina?
|
Block vascular L-type calcium channels which leads to decreased heart contractility and increased vasodilation
|
|
|
ANTIHYPERTENSIVE AGENTS
|
ANTIHYPERTENSIVE AGENTS
|
|
|
According to JNC 7 guidelines, please define the following:
Normal blood pressure |
<120/80 mm Hg
|
|
|
According to JNC 7 guidelines, please define the following:
Prehypertension |
120 to 139/80 to 89 mm Hg
|
|
|
According to JNC 7 guidelines, please define the following:
Stage I hypertension |
140 to 159/90 to 99 mm Hg
|
|
|
According to JNC 7 guidelines, please define the following:
Stage II hypertension |
≥ 160/100 mm Hg
|
|
|
What is the goal blood pressure (BP) in patients without diabetes mellitus (DM) or chronic kidney disease?
|
<140/90 mm Hg
|
|
|
What is the goal BP in patients with DM or chronic kidney disease?
|
<130/80 mm Hg
|
|
|
Define essential hypertension (HTN):
|
HTN of unknown etiology, that is, primary hypertension
|
|
|
True or False?
The majority of HTN cases are essential. |
TRUE
|
|
|
What is the BP equation?
|
Blood pressure (BP) = cardiac output (CO) × total peripheral resistance (TPR)
BP = CO x TPR |
|
|
What is responsible for moment-to-moment changes in BP?
|
Baroreceptor reflexes (autonomic nervous system)
|
|
|
Where are the baroreceptors that are sensitive to the moment-to-moment changes in BP located?
|
Aortic arch; carotid sinuses
|
|
|
What organ is responsible for the long- term control of BP?
|
Kidney
|
|
|
The kidney responds to reduced BP by releasing what peptidase?
|
Renin
|
|
|
Renin is responsible for what enzymatic reaction?
|
Conversion of angiotensinogen to angiotensin-I (AT-I)
|
|
|
What enzyme is responsible for converting AT-I to AT-II?
|
Angiotensin-converting enzyme (ACE)
|
|
|
Where is ACE found?
|
In the lungs
|
|
|
What function does AT-II have with regard to BP regulation?
|
Vasoconstriction (increases TPR thereby increases BP); stimulation of aldosterone release
|
|
|
Where is aldosterone synthesized?
|
Zona glomerulosa of the adrenal cortex
|
|
|
What function does aldosterone have with regard to BP regulation?
|
Increases reabsorption of sodium ion in exchange for potassium ion; water osmotically follows sodium ion, therefore, aldosterone leads to salt and water retention thereby increasing BP
|
|
|
What is the name of the most common thiazide diuretic used in the treatment of HTN?
|
Hydrochlorothiazide (HCTZ)
|
|
|
What are the immediate/acute effects of thiazide diuretics?
|
Increased sodium, chloride, and water excretion which leads to decreased blood volume
|
|
|
What are the chronic effects of thiazide diuretics?
|
Decreased TPR
|
|
|
What is the site of action of thiazide diuretics?
|
Distal convoluted tubule of nephron
|
|
|
What transporter (in the distal convoluted tubule) is inhibited by thiazide diuretics?
|
Na⁺/Cl transporter
|
|
|
Give examples of thiazide diuretics:
|
HCTZ
chlorothiazide chlorthalidone |
|
|
Thiazide diuretics may be ineffective in patients with creatinine clearances of less than what?
|
50 mL/min
|
|
|
With regard to blood concentrations, state whether each of the following electrolytes will be increased or decreased in patients on thiazide diuretic therapy:
Calcium |
Increased
|
|
|
With regard to blood concentrations, state whether each of the following electrolytes will be increased or decreased in patients on thiazide diuretic therapy:
Magnesium |
Decreased
|
|
|
With regard to blood concentrations, state whether each of the following electrolytes will be increased or decreased in patients on thiazide diuretic therapy:
Potassium |
Decreased
|
|
|
With regard to blood concentrations, state whether each of the following electrolytes will be increased or decreased in patients on thiazide diuretic therapy:
Sodium |
Decreased
|
|
|
With regard to increased renal calcium reabsorption, what are thiazide diuretics sometimes used for?
|
Treatment of calcium stones in the urine
|
|
|
What are the adverse effects of HCTZ?
|
• Hypercalcemia
• hypokalemia • hypomagnesemia • hyperglycemia • hyperuricemia • pancreatitis • metabolic alkalosis • Stevens-Johnson syndrome • hyperlipidemia |
|
|
Patients allergic to what class of antimicrobials may also be sensitive to thiazide diuretics?
|
Sulfonamides
|
|
|
What is the site of action of loop diuretics?
|
Loop of Henle (thick ascending limb)
|
|
|
Give examples of loop diuretics:
|
• Furosemide
• bumetanide • ethacrynic acid • torsemide |
|
|
Which loop diuretic can be given safely to patients with allergy to sulfonamide antimicrobials?
|
Ethacrynic acid
|
|
|
What transporter (in the thick ascending loop of Henle) is inhibited by loop diuretics?
|
Na⁺/K⁺/2Cl- transporter
|
|
|
True or False? Loop diuretics increase calcium excretion.
|
TRUE
|
|
|
What are the adverse effects of loop diuretics?
|
• Hypersensitivity
• hypocalcemia • hypokalemia • hypomagnesemia • metabolic alkalosis • hyperuricemia • ototoxicity |
|
|
Which loop diuretic is the most ototoxic?
|
Ethacrynic acid
|
|
|
Which renal tubular segment is responsible for the majority of sodium reabsorption?
|
Proximal convoluted tubule (>60%)
|
|
|
What is the mechanism of action of mannitol?
|
Acts as an osmotic diuretic, thereby drawing water via increased osmolality, into the proximal convoluted tubule, loop of Henle (thin descending limb), and the collecting ducts
|
|
|
What is mannitol used for?
|
Decreases intraocular and intracranial pressure; prevents anuria in hemolysis and rhabdomyolysis
|
|
|
Give two examples of carbonic anhydrase inhibitors (CAIs):
|
① Acetazolamide
② Dorzolamide |
|
|
What is the mechanism of action of CAIs?
|
Increased excretion of sodium and bicarbonate
|
|
|
What metabolic disturbance may be caused by CAIs?
|
Metabolic acidosis
|
|
|
What are CAIs used for?
|
• Altitude sickness (decreases cerebral and pulmonary edema)
• glaucoma (decreases aqueous humor formation thereby decreasing intraocular pressure) • metabolic alkalosis • to enhance renal excretion of acidic drugs |
|
|
Name three potassium-sparing diuretics:
|
① Spironolactone
② Triamterene ③ Amiloride |
|
|
What is the mechanism of action of spironolactone?
|
Aldosterone receptor antagonist
|
|
|
Where in the kidney is the aldosterone receptor found?
|
Basolateral membrane of the principal cell in the collecting duct
|
|
|
Where in the kidney does triamterene and amiloride work?
|
Sodium ion channel on the luminal side of the principal cell in the collecting duct
|
|
|
What are the adverse effects of spironolactone?
|
Hyperkalemia; metabolic acidosis; gynecomastia
|
|
|
Triamterene is often used in combination with what other diuretic?
|
HCTZ
|
|
|
Spironolactone is used to treat what conditions?
|
HTN; CHF; ascites
|
|
|
Amiloride is used to treat what conditions?
|
HTN; CHF; lithium-induced diabetes insipidus
|
|
|
With regard to BP = CO × TPR, how do β-blockers lower BP?
|
Decrease CO
|
|
|
What is the prototype β-blocker?
|
Propranolol
|
|
|
Is propranolol cardioselective?
|
No
|
|
|
Because of their cardioselectivity, what two β-blockers have gained the most widespread use?
|
① Metoprolol
② Atenolol |
|
|
Patients with what specific disease states should not receive nonselective β-antagonists?
|
Asthma (increased risk of bronchospasm); diabetes; peripheral vascular disease
|
|
|
What action do β-blockers have on the kidneys?
|
They decrease renin release by preventing stimulation of renin release by catecholamines and also likely by direct depression of the renin-angiotensin-aldosterone system.
|
|
|
What are the adverse effects of β-blockers?
|
• Hypotension
• lipid abnormalities • rebound HTN with abrupt withdrawal • fatigue • insomnia • sexual dysfunction • hallucinations • depression • hyperglycemia |
|
|
Which β-blocker has the shortest half-life?
|
Esmolol (9 min), therefore esmolol is usually administered as a continuous drip.
|
|
|
With regard to BP = CO × TPR,
how do ACE inhibitors decrease BP? |
Decrease TPR
|
|
|
In addition to conversion of AT-I to AT-II, what other reaction does ACE catalyze?
|
The breakdown of bradykinin. ACE is also known as kininase.
|
|
|
What action does bradykinin have on vascular smooth muscle?
|
Vasodilation
|
|
|
Increased bradykinin levels may lead to what adverse reaction experienced by patients who take ACE inhibitors?
|
Dry cough
|
|
|
How do ACE inhibitors decrease sodium and water retention?
|
Decreased levels of AT-II leads to decreased levels of aldosterone and therefore reduced sodium and water retention.
|
|
|
What are the adverse effects of ACE inhibitors?
|
• Hypotension
• dry cough • hyperkalemia • angioedema • fever • altered taste |
|
|
Give examples of ACE inhibitors:
|
• Benazepril
• captopril • enalapril • fosinopril • lisinopril • quinapril • ramipril |
|
|
Give two contraindications for ACE inhibitor therapy:
|
① Pregnancy (teratogenic)
② Bilateral renal artery stenosis |
|
|
What is the mechanism of action of losartan?
|
AT-II receptor blocker
|
|
|
Give examples of ARBs:
|
• Candesartan
• eprosartan • irbesartan • losartan • olmesartan • telmisartan • valsartan |
|
|
Which specific receptor type do ARBs antagonize?
|
AT-II type 1 receptor
|
|
|
Do ARBs cause dry cough?
|
No (ARBs do not inhibit the breakdown of bradykinin)
|
|
|
Is there a pregnancy contraindication for ARBs?
|
Yes, they are pregnancy category C for the first trimester and category D for the second and third trimester.
|
|
|
How do CCBs work in the treatment of HTN?
|
Block vascular L-type calcium channels which leads to decreased heart contractility and increased vasodilation of coronary and peripheral vasculature
|
|
|
What are the adverse effects of the DHP CCBs?
|
• Peripheral edema
• hypotension • reflex tachycardia • headache • flushing • gingival hyperplasia |
|
|
What are the adverse effects of verapamil?
|
• Drug-drug interactions
• constipation • AV block • headache |
|
|
What action do CCBs have on the kidney?
|
Natriuretic activity
|
|
|
Which CCB is used in subarachnoid hemorrhages to prevent vasospasm?
|
Nimodipine
|
|
|
Give an example of a T-type CCB used in the treatment of absence seizures:
|
Ethosuximide
|
|
|
Name three α₁-adrenergic antagonists used in the treatment of HTN:
|
① Doxazosin
② Prazosin ③ Terazosin |
|
|
How do α₁-adrenergic antagonists decrease BP?
|
Antagonize α₁-receptors in vascular smooth muscle, thereby causing vasodilation and reduced TPR
|
|
|
What are the two main adverse effects of α₁-antagonists?
|
① First dose syncope
② Reflex tachycardia |
|
|
What class of drugs can be used to block the reflex tachycardia caused by α₁-antagonists?
|
β-Blockers
|
|
|
α₁-Adrenergic antagonists are most commonly used for what condition other than hypertension?
|
Benign prostatic hyperplasia (BPH)
|
|
|
How does clonidine work as an antihypertensive?
|
Central α₂-adrenergic agonist (decreases sympathetic outflow from the CNS, producing a decrease in TPR, HR, and BP)
|
|
|
Why are diuretics often given to patients on clonidine therapy?
|
Clonidine causes sodium and water retention.
|
|
|
Other than HTN, what is clonidine used for?
|
• Nicotine withdrawal
• heroin withdrawal • alcohol dependence • migraine prophylaxis • severe pain • clozapine-induced sialorrhea |
|
|
What are the adverse effects of clonidine?
|
• Bradycardia
• sedation • dry mouth • rebound HTN with abrupt withdrawal (must taper down dose when discontinuing therapy) • edema |
|
|
Can clonidine be used in patients with renal dysfunction?
|
Yes, because renal blood flow and glomerular filtration rate are not compromised with clonidine therapy.
|
|
|
α-Methyldopa is converted to what active metabolite?
|
Methylnorepinephrine
|
|
|
What is the mechanism of action of α-methyldopa?
|
Central α₂-adrenergic agonist
|
|
|
Can α-methyldopa be used in patients with renal dysfunction?
|
Yes
|
|
|
Is α-methyldopa contraindicated in pregnancy?
|
No, it is one of the antihypertensive drugs of choice in pregnant women.
|
|
|
What are the adverse effects of α-methyldopa?
|
• Sedation
• hemolytic anemia • drug-induced lupus • edema • bradycardia • dry mouth |
|
|
What arterial vasodilator decreases TPR, often requires a β-blocker to counteract the subsequent reflex tachycardia, and may cause drug-induced lupus?
|
Hydralazine
|
|
|
What class of drugs are used to counteract the fluid retention caused by hydralazine?
|
Diuretics
|
|
|
What are the adverse effects of hydralazine?
|
• Reflex tachycardia
• fluid retention • drug-induced lupus • headache • flushing |
|
|
What enzyme is responsible for the metabolism of hydralazine?
|
N-acetyltransferase
|
|
|
What is the mechanism of action of minoxidil?
|
Opens potassium channels which leads to membrane hyperpolarization and subsequent arterial vasodilation
|
|
|
Minoxidil is a prodrug that is activated by what mechanism?
|
Sulfation
|
|
|
What is a common side effect of minoxidil?
|
Hypertrichosis (used in the treatment of alopecia)
|
|
|
What drug is a direct acting vasodilator, used in hypertensive emergencies, and can also be used to prevent hypoglycemia in patients with insulinoma?
|
Diazoxide
|
|
|
How does diazoxide work to prevent hypoglycemia in patients with insulinoma?
|
It decreases insulin release.
|
|
|
What drug is used during hypertensive emergencies, acts via increasing cGMP through NO mechanisms, and forms thiocyanate and cyanide metabolites?
|
Sodium nitroprusside
|
|
|
What class of antihypertensives may help prevent left ventricular remodeling seen in patients with CHF?
|
ACE inhibitors
|
|
|
What class of antihypertensives may improve patients’ lipid profiles?
|
α₁-Adrenergic antagonists
|
|
|
What two classes of antihypertensives may protect renal function in patients with DM?
|
ACE inhibitors and ARBs by decreasing microalbuminuria
|
|
|
ANTIHYPERLIPIDEMIC AGENTS
|
ANTIHYPERLIPIDEMIC AGENTS
|
|
|
What is the mechanism of action of the 'statin' class of antihyperlipidemics?
|
Inhibition of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase (rate-limiting step of cholesterol biosynthesis), thereby inhibiting the intracellular supply of cholesterol; increases number of cell surface low-density lipoproteins (LDL) receptors which further reduces plasma cholesterol levels via increased metabolism
|
|
|
How do statins affect the lipid profile?
|
Mainly reduces LDL; moderate reduction of triglycerides (TGs); moderate elevation of high-density lipoproteins (HDL)
|
|
|
Give examples of medications in the statin drug class:
|
• Atorvastatin
• rosuvastatin • simvastatin • pravastatin • lovastatin • fluvastatin |
|
|
What are the adverse effects of statin drugs?
|
• Increased liver function tests (LFTs)
• dose-dependent rhabdomyolysis, myalgia, and myopathy • diarrhea • drug-induced lupus • drug-drug interactions • contraindicated in pregnancy (category X) |
|
|
What laboratory tests should be used to monitor for statin toxicity?
|
LFTs; creatine phosphokinase (CPK)
|
|
|
What is the most potent antihyperlipidemic agent to elevate high-density lipoprotein (HDL)?
|
Niacin
|
|
|
Niacin is also known as what?
|
Vitamin B3; nicotinic acid
|
|
|
What is the mechanism of action of niacin?
|
High-dose niacin inhibits very low-density lipoprotein (VLDL) and apoprotein synthesis in hepatocytes; increases tissue plasminogen activator (tPA); decreases fibrinogen
|
|
|
How does niacin affect the lipid profile?
|
• Elevation of HDL
• reduction of LDL • reduction of VLDL • reduction of TGs |
|
|
What are the adverse effects of niacin?
|
• Facial flushing
• hyperuricemia • hyperglycemia • hepatotoxicity • pruritus • nausea • abdominal pain |
|
|
How is facial flushing counteracted in patients on niacin therapy?
|
Pretreatment with aspirin to counteract the prostaglandin release
|
|
|
What new antihyperlipidemic agent works by inhibiting absorption of cholesterol at the brush border in the small intestine?
|
Ezetimibe
|
|
|
What are the adverse effects of ezetimibe?
|
• Diarrhea
• abdominal pain • headache • arthralgias |
|
|
Which antihyperlipidemic drug class decreases VLDL, LDL, TGs and increases HDL by activating lipoprotein lipase?
|
Fibrates
|
|
|
Give examples of medications in the fibrate drug class:
|
Gemfibrozil; clofibrate; fenofibrate
|
|
|
What is the major effect that fibrates have on the lipid profile?
|
Reduction of TGs
|
|
|
What are the adverse effects of gemfibrozil?
|
• Cholelithiasis
• nausea • diarrhea • abdominal pain • myositis • hypokalemia • increased levels of warfarin and sulfonylureas via protein-binding displacement |
|
|
What is the mechanism of action of bile acid sequestrants?
|
Anion exchange resins that bind bile acids/salts in the small intestine, thereby preventing their reabsorption; decreased bile acids results in increased plasma cholesterol uptake by hepatocytes to replenish bile acid levels, thereby decreasing plasma LDL levels
|
|
|
Give examples of bile acid sequestrants:
|
Cholestyramine; colestipol; colesevelam
|
|
|
What are the adverse effects of bile acid sequestrants?
|
• Constipation
• bloating, flatulence • nausea • impaired absorption of fat-soluble vitamins impaired absorption of • warfarin • digoxin • acetyl salicylic acid (ASA) • Tetracycline (TCN) • thiazide diuretics |
|
|
What over-the-counter medication, naturally found in certain fish, can decrease TG levels and be useful in the prevention of coronary heart disease (CHD)?
|
Omega-3 fatty acids
|
|
|
ANTICOAGULATION AGENTS
|
ANTICOAGULATION AGENTS
|
|
|
Where is prostacyclin made?
|
Vascular endothelial cells
|
|
|
Where is thromboxane A2 made?
|
Platelets
|
|
|
Increased prostacyclin leads to an increase of what second messenger?
|
cAMP
|
|
|
cAMP does what to platelets?
|
Inhibits platelet activation and release of platelet aggregating factors
|
|
|
How does ASA work as a platelet aggregation inhibitor?
|
Irreversible inhibition, via acetylation, of cyclooxygenase (COX) thereby reducing thromboxane A2 levels
|
|
|
What two drugs inhibit platelet aggregation and platelet-fibrinogen interaction by blocking adenosine diphosphate (ADP) receptors?
|
① Clopidogrel
② Ticlopidine |
|
|
What are the major adverse effects of ticlopidine?
|
Neutropenia; agranulocytosis; thrombotic thrombocytopenic purpura (TTP)
|
|
|
How should patients on ticlopidine therapy be monitored?
|
Complete blood count (CBC) with differential every 2 weeks for the first 3 months of therapy and as needed thereafter
|
|
|
How does dipyridamole work as a platelet aggregation inhibitor?
|
Inhibits phosphodiesterase (PDE) thereby increasing cAMP levels; cAMP potentiates prostacyclin and inhibits thromboxane A2 synthesis
|
|
|
Dipyridamole is usually given in combination with what drug?
|
Aspirin
|
|
|
What are the two main systems that feed into the common pathway of the clotting cascade?
|
① Intrinsic system
② Extrinsic system |
|
|
Activation of clotting factor VII to VIIa marks the beginning of which clotting system?
|
Extrinsic system
|
|
|
Activation of clotting factor XII to XIIa marks the beginning of which clotting system?
|
Intrinsic system
|
|
|
Activation of which clotting factor marks the beginning of the common pathway?
|
Activation of factor X to factor Xa
|
|
|
What is another name for factor II? What is another name for factor IIa?
|
Prothrombin
|
|
|
What factor is also known as 'Christmas factor'?
|
Thrombin
|
|
|
What factor is deficient in hemophilia A?
|
Factor IX
|
|
|
What factor is deficient in hemophilia B?
|
Factor VIII Factor IX
|
|
|
How does heparin work as an anticoagulant?
|
Complexes with antithrombin-III to increase inactivation of clotting factors IIa, IXa, Xa, XIa, XIIa, and XHIa
|
|
|
Which system of the clotting cascade is mainly affected by heparin?
|
Intrinsic system
|
|
|
Which laboratory test is used to monitor heparin therapy?
|
Partial thromboplastin time (PTT); therapeutic PTT levels are 1.5 to 2.5 times that of normal
|
|
|
How is heparin administered?
|
Intravenously (IV), subcutaneously (SQ)
|
|
|
Why is intramuscular administration of heparin contraindicated?
|
Hematoma formation
|
|
|
What are the therapeutic indications of heparin?
|
Prevention and treatment (by preventing expansion of the clot) of deep vein thrombosis (DVT) and pulmonary embolism (PE); used for anticoagulation during MI
|
|
|
What is the onset of action of heparin?
|
IV = immediate onset of action; SQ = 20 to 30 minutes
|
|
|
Does heparin cross the placental barrier?
|
No, therefore it is safe in pregnancy.
|
|
|
What are the adverse effects of heparin?
|
• Bleeding
• hypersensitivity • osteoporosis • heparin-induced thrombocytopenia (HIT) |
|
|
Heparin undergoes what type of metabolism?
|
Hepatic; reticuloendothelial system
|
|
|
What drug is used to counteract heparin overdose?
|
Protamine sulfate
|
|
|
Roughly, how many units of heparin are neutralized by 1 mg of protamine sulfate?
|
100 units
|
|
|
What is the onset of action of protamine sulfate?
|
5 minutes
|
|
|
What paradoxical adverse effect can protamine cause at high doses?
|
Anticoagulation leading to hemorrhage
|
|
|
What is the mechanism of action of enoxaparin?
|
Low-molecular-weight heparin (LMWH) that has a higher ratio of antifactor Xa to antifactor Ha activity versus unfractionated heparin (UFH)
|
|
|
What is the half-life of LMWH?
|
Two to four times that of UFH
|
|
|
Does PTT need to be monitored in patients on LMWH therapy?
|
No
|
|
|
What synthetic pentasaccharide causes an antithrombin-III-mediated selective inhibition of factor Xa?
|
Fondaparinux
|
|
|
Which system of the clotting cascade is mainly affected by warfarin?
|
Extrinsic system
|
|
|
Which laboratory tests are used to monitor warfarin therapy?
|
Prothrombin time (PT); international normalized ratio (INR) → therapeutic INR levels are between 2 and 3
|
|
|
How does warfarin work as an anticoagulant?
|
Inhibits synthesis of vitamin-K-dependent clotting factors II, VII, IX, and X via inhibition of vitamin K epoxide reductase; also inhibits synthesis of protein C and protein S
|
|
|
What is the onset of action of warfarin?
|
36 to 72 hours (anticoagulant action)
|
|
|
How long after initiation of warfarin therapy is the full therapeutic effect seen?
|
5 to 7 days (antithrombotic action)
|
|
|
Acute alcohol intoxication does what to warfarin metabolism?
|
Inhibits warfarin metabolism, thereby increasing warfarin blood levels
|
|
|
Chronic alcohol use does what to warfarin metabolism?
|
Induces warfarin metabolism, thereby decreasing warfarin blood levels
|
|
|
What happens to the INR of warfarin patients who begin thyroid replacement medication?
|
INR increases; thyroid hormone increases the metabolism of clotting factors, thereby potentiating the effects of warfarin
|
|
|
What happens to the INR of warfarin patients who begin antimicrobial therapy with sulfonamides?
|
INR increases; sulfonamides inhibit CYP-450 2C9, thereby increasing warfarin levels
|
|
|
Does warfarin cross the placental barrier?
|
Yes (contraindicated in pregnancy); warfarin is teratogenic
|
|
|
What are the adverse effects of warfarin?
|
• Bleeding
• drug-drug interactions • skin necrosis (seen within the first few days of warfarin therapy and is secondary to decreased protein C levels) • 'purple toes syndrome' (caused by cholesterol microembolization) |
|
|
What can be used to counteract the effects of warfarin?
|
Vitamin K (slow onset); fresh frozen plasma (rapid onset)
|
|
|
How is warfarin metabolized?
|
Hepatic cytochrome β-450 enzymes
|
|
|
The synthesis of what two factors is inhibited first when warfarin therapy is initiated?
|
① Factor VII
② Protein C (therefore patients may initially be hypercoagulable when warfarin is first initiated) |
|
|
Why are factor VII and protein C inhibited first when warfarin therapy is initiated?
|
These proteins have the shortest half-lives when compared to the half-lives of factors II, IX, X, and protein S
|
|
|
What is the mechanism of action of abciximab, eptifibatide, and tirofiban?
|
Blockade of the glycoprotein IIb/IIIa receptor on platelets, thereby inhibiting platelet aggregation
|
|
|
What is the physiologic ligand for the glycoprotein Ilb/IIIa receptor?
|
Fibrinogen
|
|
|
What is the mechanism of action of thrombolytic agents?
|
Conversion of plasminogen to plasmin; plasmin cleaves fibrin, thereby leading to lysis of thrombi
|
|
|
What is the main adverse effect of thrombolytic agents?
|
Hemorrhage
|
|
|
Thrombolytic agents are contraindicated in what settings?
|
• Active internal bleeding
• history of cerebrovascular accident (CVA) • recent intracranial or intraspinal surgery • intracranial neoplasm • AV malformation • severe uncontrolled HTN (systolic blood pressure [SBP] >185 mm Hg or diastolic blood pressure [DBP] >110 mm Hg) • evidence of intracranial hemorrhage • suspected aortic dissection • seizure at the onset of stroke • current use of anticoagulants or an INR >1.7 • lumbar puncture within 1 week |
|
|
What are the therapeutic indications of thrombolytic therapy?
|
Acute MI; acute PE; acute ischemic stroke
|
|
|
What is the 'therapeutic time window' for administering thrombolytic agents to patients with acute ischemic stroke?
|
Within the first 3 hours of the onset of symptoms
|
|
|
Give examples of thrombolytic agents:
|
• Alteplase
• anistreplase • streptokinase • urokinase |
|
|
What two drugs can counteract thrombolytic agent therapy?
|
① Aminocaproic acid
② Tranexamic acid (both agents inhibit plasminogen activation) |
|
|
With regard to thrombolytic agents, what does 'clot-specific' mean?
|
The drug specifically activates plasminogen that is bound to fibrin in a thrombus with a low affinity for free, circulating plasminogen
|
|
|
Which thrombolytic agent is 'clot-specific'?
|
Alteplase
|
|
|
Alteplase is also known as what?
|
tPA (tissue plasminogen activator)
|
|
|
What is the half-life of tPA?
|
5 minutes
|
|
|
Where does tPA come from?
|
Recombinant DNA technology
|
|
|
What type of enzymatic activity does tPA possess?
|
Serine protease activity
|
|
|
Where does streptokinase come from?
|
Group C β-hemolytic streptococci
|
|
|
How does streptokinase work as a thrombolytic agent?
|
Forms a 1:1 complex with plasminogen; complexed plasminogen then converts free plasminogen into plasmin (active form)
|
|
|
Does streptokinase have any enzymatic activity?
|
No
|
|
|
Is streptokinase 'clot-specific'?
|
No
|
|
|
What other proteins does the streptokinase-plasminogen complex degrade?
|
Fibrinogen; factor V; factor VII
|
|
|
What laboratory value is monitored with streptokinase therapy?
|
Thromboplastin time
|
|
|
Why is streptokinase antigenic?
|
It is recognized as a foreign protein (antigen).
|
|
|
What adverse reactions are specific to streptokinase?
|
Anaphylaxis; rash; fever
|
|
|
What is the half-life of anistreplase?
|
90 minutes
|
|
|
How does anistreplase work as a thrombolytic?
|
Anisoyl group blocks the active site of plasminogen; as complex binds to fibrin, anisoyl group is removed and the complex becomes activated.
|
|
|
Does urokinase have enzymatic activity?
|
Yes
|
|
|
Originally, where did urokinase come from?
|
Human urine
|
|
|
Where does urokinase come from now?
|
Fetal renal cells (human)
|
|
|
Is urokinase antigenic?
|
No, since it is not a foreign protein.
|
|
|
Will streptokinase, at normal doses, work in patients with a recent history of streptococcal infection?
|
No, because antibodies made against recent streptococcal antigens will bind to and inactivate streptokinase.
|
|
|
CLINICAL VIGNETTES
|
CLINICAL VIGNETTES
|
|
|
A 72-year-old man with a past medical history of CHF presents to the emergency room with colicky right-sided flank pain. Renal ultrasonography shows a 5 mm calculus in the right ureter. What medication is the patient likely on that has exacerbated this situation? What medication could the patient be switched to?
|
Furosemide and other loop diuretics are frequently employed in the treatment of CHF to help decrease preload to the heart and vascular congestion that can cause pulmonary edema. However, loop diuretics increase the urinary excretion of calcium which can lead to renal stone formation. As this patient is presenting with a stone, if his CHF symptoms can be controlled on an alternative agent, the loop diuretics should be discontinued. A thiazide diuretic, such as hydrochlorothiazide, may provide adequate diuresis to control his CHF symptoms, while at the same time decreasing urinary calcium excretion, preventing further stone formation.
|
|
|
A 32-year-old woman is found to have a deep venous thrombosis (DVT) in her right calf and therapy is started with heparin bridging therapy with close monitoring of her activated partial thromboplastin time. Warfarin is added to the therapy, with the goal of discontinuing heparin once the patient reaches a therapeutic INR. A repeat Doppler ultrasonography of the right calf after 1 week of therapy unexpectedly shows expansion of the clot. What is likely to be found on this patient’s complete blood count (CBC), and what is the appropriate therapy?
|
Paradoxical clot expansion or new clot formation is most likely the result of heparin-induced thrombocytopenia (HIT) in this patient. HIT is a rare complication seen after at least 7 days of treatment with unfractionated heparin. Heparin molecules induce the formation of antiheparin antibodies (IgG). These antibodies bind to heparin and platelet factor 4, causing platelet activation and aggregation, with a subsequent drop in platelet numbers. Therefore, the CBC will reveal a low platelet count. Heparin therapy should be stopped immediately. Low-molecular-weight heparins such as enoxaparin are contraindicated in patients with a history of HIT, and therefore would not be appropriate alternative therapy in this patient. Here, we could measure the patient’s INR, and if therapeutic, warfarin monotherapy could be continued for 3 to 6 months. Alternative anticoagulation therapies available to patients with HIT include lepirudin, a factor Ha inhibitor, and argatroban, a synthetic direct thrombin inhibitor.
|
|
|
A 63-year-old man who is being treated for hypercholesterolemia with an HMG-CoA reductase inhibitor (a statin) comes in for a follow-up visit. He began taking the medication 1 week ago. He is complaining of some mild muscle aches and pains, which he attributes to the new exercise regimen he just started on your recommendation. Laboratory studies show a minor increase in creatine kinase (CK) activity. He asks if the new medication could be causing this pain, and if he should stop taking the medication. How should the physician advise the patient?
|
Statin myopathy is a concerning but relatively rare complication of statin therapy, with an incident of 0.1% to 0.5%. Rhabdomyolysis due to statin therapy is notably less common. Rhabdomyolysis is an indication for immediate cessation of statin therapy, but myopathy is not necessarily. Myopathy usually manifests within 1 week of starting a statin, and is dose dependent. If statin therapy is successful in lowering serum cholesterol, and CK elevation is not significantly elevated above baseline, lowering the dose of the medication or switching to a different statin may be appropriate. A controversial topic is the use of coenzyme Q10 (CoQIO), or ubiquinone, to treat and prevent statin-induced myopathy. Statins also inhibit the reaction that forms CoQIO. CoQIO is found in the mitochondria of many cell types and is an antioxidant that might maintain muscle health. It has been shown in limited studies to decrease muscle pain in patients taking statins. Its long-term safety and efficacy are yet unknown.
|

