![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
110 Cards in this Set
- Front
- Back
|
How can the decreased volume in the kidney translate low [drug]in the blood to high [drug] in the kidney?
|
180 L / day goes thru kidney
1 L / day excreted in urine |
|
|
Toxic nephropathy
|
a general term which describes renal damage caused by either exogenous or endogenous compounds leading to renal dysfunction
important because it is a reversible renal injury if detected early |
|
|
Renal damage
|
can be due to several different mechanisms affecting different segments of the nephron, renal microvasculature or interstitium
|
|
|
Stressed Kidney
|
-Useful concept in clinical practice.
- Any kidney where GFR is sustained by compensatory mechanisms (PGs, Angiontensin II can be considered as stressed. - These include: Hypovolaemia, cardiac failure, pre-existing renal disease, hepatic dysfunction, renal vascular disease and salt depletion, patients in diuretic or cyclosporin therapy, and elderly. |
|
|
Diagnosing Renal Damage
|
* Clinical signs may not be apparent in the early stages.
* Assessment of renal function should include thorough evaluation of glomerular filtration rate (GFR), proximal and distal tubular function. * A kidney biopsy may be indicated to establish the cause and effect relationship. * SCr is a biomarker for kidney malfunction If not excreted your filter is not working well |
|
|
Drugs commonly causing acute tubular necrosis
|
- Aminoglycoside antibiotics
- Amphoterecin B - Acetaminophen - Cisplatin - Tetracycline - Cephalosporins |
|
|
High Drug Levels in Kidney
|
- Kidney receives 25 % of the cardiac output (kidney cortex –proximal tubule).
- One function of the kidney is to concentrate materials for efficient excretion with minimal loss of fluid. - Concentration primarily occurs in the proximal tubule and changes would be expected first in cells of the proximal nephron. |
|
|
Metabolic Capacity: Cytochrome P450
|
About 25% of the liver. Predominately in cortex low or absent in medulla/papilla (acetaminophen).
Cortex = high Medulla/papilla = low |
|
|
Metabolic Capacity: GSH and related enzymes
|
.Gradient from Cortex (high) to Medulla (low)
GSH low in Medulla = more toxicity in Medulla |
|
|
Metabolic Capacity: Prostaglandin H synthase
|
(very high in medulla/papilla ). (dilators)
|
|
|
Acute Renal Dysfunction
|
- An episode of acute deterioration in renal function may vary in sensitivity from small asymptomatic rise in serum creatinine level to renal failure requiring support and dialysis.
- The mechanism may also vary. - Drug induced acute renal failure accounts up to 20% of all such cases, but the incidence of microepisodes is uncertain. - It has been suggested that 15% of patients in an intensive care unit will develop drug-induced acute renal failure. (very important) |
|
|
Drugs that can cause Pre-renal Failure
|
NSAIDs
ACEi's Cyclosporin A |
|
|
The effects of NSAIDs on renal function
|
- Decline in renal condition may be severe but more likely is mild and asymptomatic. Situation reverts back to normal after withdrawal of the drug.
- The acute reversible effects of NSAIDs on renal function due to inhibition of renal prostaglandins are well understood. - COX2 is constitutively expressed in kidney - COX2 plays a role in renal development - Case reports of renal disease by COX2 inhibitors |
|
|
Amphoterecin B (renal)
|
- A very effective antifungal drug whose clinical utility is limited by its nephrotoxicity.
- One study reported impairment in up to 80% of patients exposed to a therapeutic dose. - Toxicity is dose related and predictable in doses of greater than 5 g. - Alters membrane permeability and induces renal vasoconstriction. - Use of liposomes seem to decrease toxicity. |
|
|
Acetaminophen (renal)
|
Although uncommon, it is possible to have acute renal failure due to acetaminophen toxicity in the absence of fulminant hepatic failure.
|
|
|
Prevention of Drug-induced Acute Kidney Injury
|
(a) Look for the presence of risk factors for nephrotoxicity.
(b) Use alternative therapies for drugs with potential nephrotoxicity. (c) Use appropriate drug dosing adapted to altered kinetics. (d) the correct assessment of kidney function before and during treatment with the aim of early recognition of kidney injury, and (e) Use preventive measures for nephrotoxicity (general and specific). |
|
|
With respect to electrophiles which statement is FALSE?
a) Electrophiles may react with nucleophilic sites of DNA. b) Electrophiles may react with the -SH sites of proteins. c) Cell division is not sensitive to electrophiles. d) Electrophiles are electron-deficient compounds. |
c) Cell division is not sensitive to electrophiles.
|
|
|
Which statement is TRUE?
a) Lipid peroxidation does not affect cell membranes. b) Lipid peroxidation detoxifies toxic aldehydes. c) Vitamin E enhances lipid peroxidation d) Free radical may initiate lipid peroxidation |
d) Free radical may initiate lipid peroxidation
|
|
|
Drug metabolism catalyzed by cytochrome P-450s leads to the formation:
1) oxygenated intermediates that are not reactive molecules. 2) conjugated water soluble products. 3) oxygenated highly reactive intermediates. 4) conjugated compounds that are potent electrophiles |
3) oxygenated highly reactive intermediates.
|
|
|
With respect to Phase II enzymes of drug metabolism which of the following statement is TRUE:
1) Glucuronidation is catalyzed by epoxide hydrolases. 2) Glutathione can react with electrophilic drug metabolites only in the presence of glutathione-S- transferases. 3) Sulfate esters are the products of sulfation. 4) Both sulfation and glucuronidation form hydrophilic metabolites |
3) Sulfate esters are the products of sulfation.
|
|
|
Metabolism of acetaminophen to its reactive metabolite N-acetyl-p-benzoquinone imine, which is a potent electrophile, involves the following enzymes:
1) CYP2E1 2) Aldehyde dehydrogenase and CYP1A2 3) CYP1A2 and CYP2E1 4) Sulfotransferase and Glutahione-S-transferase 5) CYP2E1 and Glutahione-S-transferase |
1) CYP2E1
|
|
|
With respect to developmental toxicity, which statement is false?
a) The embryo usually has a greater susceptibility to chemicals than adults do. b) Teratogenic induction is a multicellular phenomenon. c) All teratogens that are lethal also cause malformations. d) There is usually a threshold dose that is necessary to cause a teratogenic outcome. |
c) All teratogens that are lethal also cause malformations.
|
|
|
Which statement is false?
a) If one prescribes a drug during pregnancy, FDA category A drugs are preferred. b) FDA category drugs B and C may be used by the pregnant patient. c) FDA category D drugs may be acceptable to use in the pregnant patient depending on the risk associated with the condition. d) If one prescribes a drug during pregnancy, FDA category X are preferred. |
d) If one prescribes a drug during pregnancy, FDA category X are preferred.
|
|
|
Which of the following drugs are not human teratogens?
a) Diethylstilbestrol b) Lithium c) Bendectin d) Cocaine e) All of above are human teratogens. |
c) Bendectin
|
|
|
Which statement is false?
a) The type of thalidomide-induced malformations varied depending on the developmental day of exposure. b) The most thalidomide sensitive period is between 27-40 days. c) The FDA denied thalidomide release in the USA because of teratogenic concerns. d) Humans are the most sensitive mammal to thalidomide-mediated malformations. e) None of the above |
c) The FDA denied thalidomide release in the USA because of teratogenic concerns.
|
|
|
c) The FDA denied thalidomide release in the USA because of teratogenic concerns.
|
c) ACE inhibitors such as captopril present the greatest risk in the 1st trimester.
|
|
|
Which of the following drugs may cause steatosis (fatty liver)
a) Acetaminophen b) Bleomycin c) Adriamycin. d) Tetracycline. |
d) Tetracycline.
|
|
|
Which of the following conditions can induce a stressed-kidney:
a) Cardiac failure. b) Prexisting renal disease. c) Cyclosporin therapy. d) All of the above. |
d) All of the above.
|
|
|
Non-steroidal anti-inflammatory drugs (NSIDS) can cause renal toxicity because:
a) They inhibit the synthesis of prostaglandins b) They increase the synthesis of prostaglandins. c) They inhibit angiontensin II formation. d) They increase the levels of angiontensin II. |
a) They inhibit the synthesis of prostaglandins
|
|
|
Aminogyosides (antibiotics) may cause:
a) Liver cirrhosis. b) Acute tubular necrosis. c) Reyes syndrome. d) Liver tumors. |
b) Acute tubular necrosis.
|
|
|
Cisplatin is nephrotoxic because:
a) It accumulates mainly in the kidneys. b) Kidney is responsible for the majority of cisplatin excretion. c) All of the above. d) None of the above. |
c) All of the above.
|
|
|
NAPQI, the toxic metabolite of acetaminophen, is detoxified primarily by which of the following pathways?
A. Conjugation with glucuronide B. Conjugation with glutathione C. Conjugation with sulfate D. Conjugation with N-acetylcysteine E. Oxidation by the P450 enzyme system |
B. Conjugation with glutathione
|
|
|
What is drug recall?
|
* A FDA drug recall is when the FDA determines that a drug is defective or possibly harmful.
* The FDA notifies the company that manufactures the medication to remove it from the market either temporarily (drug recall) or completely (drug withdrawal). * Usually, the company will comply; if not the FDA can seek a court order authorizing the federal government to seize the product. * In most cases, recalls are done voluntarily by the manufacturer or the distributor of the product |
|
|
What are the classes of drug recall?
|
* Class I - recalls are for dangerous or defective products that predictably could cause serious health problems or death
* Class II - recalls are for products that might cause a temporary health problem, or pose only a slight threat of a serious nature * Class III - recalls are for products that are unlikely to cause any adverse health reaction, but that violate FDA regulations |
|
|
What are undesired effects of a drug?
|
effects other than the therapeutic effect that can be
|
|
|
Define adverse effects
|
Deleterious toxic effects - fall into three categories
*pharmacological *pathological *genotoxic |
|
|
Define side effects
|
undesirable effects that are non-deleterious
|
|
|
Define toxicology
|
Toxicology is the science of the adverse effects of chemicals on living organisms.
Discipline is divided in several areas: a. Descriptive: Toxicity tests that can be used to evaluate the risk of exposure of humans to to particular chemical. b. Mechanistic: Attempts to determine how chemicals exert their deleterious effects on organisms. c. Regulatory: Judges whether a drug has low enough risk to justify making it available to humans. |
|
|
Dose-Response Relationship
|
* Toxicity depends on the dose and type of substance, the frequency of exposure and the organism in question
* Dose: The quantity of the compound received by the organism * Evaluation of dose-response or dose-effect is crucially important |
|
|
What are the characteristics of exposure?
|
Route and site of exposure
The major pathways by which toxic agents gain access to the body are: 1. The gastrointestinal tract---Ingestion. 2. Lungs—Inhalation. 3. Skin--Topical, percutaneous, or dermal. 4. Other parenteral (Other than intestinal canal). |
|
|
What is selective toxicity?
|
A drug may affect a particular cell type because of uptake, metabolism or inherent properties of the cell.
Analgesics - Concentration in the kidney. Acetaminophen - Liver damage due to metabolism to reactive intermediates. Majority of metabolism occurs in the liver |
|
|
What factors affect toxicity?
|
ADME - (species, strain, sex, age, environment, diet, disease, and genetic factors contribute to differences in ADME)
Difference in disposition Differences in toxic response |
|
|
How does absorption affect toxicity?
|
* Dermal, oral, inhalation, parenteral, ip, im, iv, sc.
* Differences in absorption will result in differences in disposition * Rate of absorption: Depends on lipid solubility (log P) and % ionization |
|
|
How does distributin affect toxicity?
|
The concentration of compound in the plasma and the plasma profile reflects the distribution.
* Compounds distributed into all tissues (e.g. many solvents) will have low plasma concentrations and high volume of distribution. * Substances which are ionized at the pH of the plasma will not distribute well, have low volume of distribution and high plasma concentrations. |
|
|
How does biotransformation affect toxicity?
|
* Taking a lipophilic drug and making it more hydrophilip in preparation for elimination
* Drugs can be metabolized by phaze 1 enzymes to active oxygenated intermediates * Intermediates can covalently bind to proteins causing injury (necrosis), hapten (antigen), or mutation (cancer) * Intermediates can be furthur metabolized by phase 2 enzymes to conjucated innocuos products which can then be eliminated |
|
|
What are the key organs involved in biotransformation and what are their relative capacities?
|
Liver = high capacity
Lung = medium capacity Kidney = medium capacity Intestine = medium capacity Skin = Low capacity testis = low capacity placenta = low cpacity adrenals = low capacity |
|
|
How does elimination affect toxicity?
|
Person to person variation in drug elimination impacts the half-life and accumulation of the drug.
|
|
|
How does excretion affect toxicity?
|
In simple terms the more rapidly compounds are excreted, the less likely they are to accumulate and exert toxic effects
Major routes Urine—H20 soluble Bile Lungs- Volatile substances Secretion into GI tract Secretion into body fluids |
|
|
Utility of Studying Mechanisms of Toxicity
|
* Prediction of drug toxicity and synthesis of non-toxic analogs.
* Design of antidotal therapies. * Health risk and regulatory limits. * Monitoring of exposed population. * Elucidation of routes of selective toxicity. * Details on the cause/progression of disease state. |
|
|
Define electrophile
|
Electrophiles: Agents that are electron deficient
(nonionic or cationic) e.g.: ketones, epoxides, a,b-unsaturated ketones and aldehydes, quinones and quinoneimines Produced: During the drug oxidations Toxicological Importance: They permanently alter endogenous molecules |
|
|
What can electrophiles do to macromolecules?
|
-DNA
*EP react with the nucleophilic sites in DNA e.g., deoxyguanosine (O6 or N7 in guanine). *The phosphate backbone is also susceptible to electrophilic attack. -Proteins *EP react with the nucleophilic sites in proteins e.g., -SH, -NH2 *This leads to the inactivation of proteins. |
|
|
Define Free Radical
|
What is it?
It is a compound with an unpaired electron. How is produced? By enzymes which catalyze 1e- oxidation/reduction reactions, by photolysis or radiation. Toxic consequences: Interaction with DNA. Oxidative damage. Lipid peroxidation. |
|
|
How are free radicals formed?
|
Formed from neutral molecules through: (a) release of one electron producing a cation free radical or (b) uptake of one electron producing an anion free radical, or (c) homolytic cleavage of a covalent bond producing a neutral radical
|
|
|
What are fenton reactions?
|
hydrogen peroxide is converted into a hydroxyl radical and OH-
|
|
|
Name at least two drugs that cause toxicity through free radicals.
|
Alloxan, Adriamycin, Bleomycin, and Paracetamol (acetaminophen)
|
|
|
What are the toxic consequences of the free radicals?
|
Interaction with DNA
Oxidative damage Lipid peroxidation |
|
|
What is redox-cycling and what is the cellular damage associated with it?
|
Reduction by a reductase followed by oxidation by molecular oxygen (dioxygen) is known as redox-cycling and continues until the system becomes anaerobic. The consequence of these enzymatic reductions is
that the semiquinone yields its extra electron to oxygen with the formation of superoxide radical anion and the original quinone. Radicals can react with nucleic acids resulting in their damage (cytostatic drugs e.g., adriamycin metabolism generates hydroquinone that binds DNA). Redox cycling* caused by several drugs (e.g., bleomycin) that can damage DNA in two ways: a. Hydroxyl radicals react with sugar-phosphate backbone of nucleic acids resulting in DNA breaks. b. ROS (reactive oxygen species) can cause oxidative damage. |
|
|
Define lipid peroxidation and its consequences.
|
Lipid peroxidation is a chain reaction of free radical with lipids.
Consequences of Lipid Peroxidation: * Chain reaction of PUFA and cholesterol. *Destroys cell membranes and leads to functional impairment of membranes bound proteins (eg. glucose 6-phosphatase). * Generates aldehydes (malondialdehyde and 4-hydroxynonenal). |
|
|
Protein modification by lipid peroxidation products.
|
WHAT??
There is one slide in lecture #1 for this one. I don;t know what it means... |
|
|
What is the ideal protective mechanism?
|
The ideal protective mechanism is excretion!
|
|
|
Conjugation of nucleophilic and electrophilic drug metabolites
|
|
|
|
Conjugation of nucleophilic and electrophilic drug metabolites
|
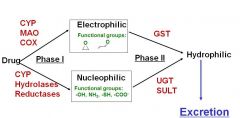
|
|
|
What are sulfation, gucuronidation, and what do they protect?
|
i) Sulfation (via sulfotransferases; SULT)
ii) Glucuronidation (via UDP-glucuronyltransferases; UGT) iii) Reaction with glutathione(via Glutathione-S transferases; GST) They protect against cellular damage resulting from reactive drug metabolites |
|
|
What are protection mechanisms against reactive species?
|
Drug metabolites
*Generation of Polar Metabolites * Enzymes (e.g, ALDH, NQO1) Free radicals *Enzymatic antioxidants * Non Enzymatic antioxidants * Metal sequestering agents * DNA-repair |
|
|
Define glutathione and its reactions with drug metabolites.
|
Glutathione: (g-L-glutamyl-L-cysteinylglycine; GSH).
* Plays a central role in cellular defense. * Occurs intracellularly at high concentration e.g. human erythrocytes contain 2 mM and hepatocytes more than 5 mM. Chemical reactions: * Direct conjugation via addition * Direct conjugation via displacement Enzymatice reactions *Via Glutathione-S transferases (GSTs): Present in high concentrations * Cytosolic or membrane bound * Membrane bound GSTs are found in ER and outer mitochondrial membrane * Glutathione conjugates may be excreted: a) In the bile as unchanged, or b) Undergo metabolism to mercapturic acid, which is excreted in urine, e.g., naphtalene |
|
|
What is the concentration of GSH in tissues?
|
Occurs intracellularly at high concentration e.g. human erythrocytes contain 2 mM and hepatocytes more than 5 mM.
|
|
|
Describe the actions of NQO1 and ALDHs and why they are important enzymes.
|
NADPH-quinone Oxidoreductase = Redox Cycling
Aldahyde Dehydrogenases reduce an alcohol to a carboxilic acid |
|
|
What are the enzymatic antioxidants?
|
i) Superoxide dismutase, Catalase, Glutathione peroxidase
iii) GSH peroxidase/GSSG reductase system |
|
|
What are the non-enzymatic antioxidants?
|
i) Vitamin E
ii) Vitamin C iii) Others |
|
|
What are the metal sequestering agents and what characteristics should have?
|
* Chelation: Is the formation of a metal complex.
* Ideal chelating agents should: * be water soluble, resistant to metabolism, able to reach sites of metal storage. * capable of forming non-toxic complexes with the metals. * have low affinity for essential metals, particularly calcium and zinc. Antibiotic drugs of the tetracycline family are chelators of Ca2+ and Mg2+ ions. |
|
|
DNA damage
|
DNA damage can be induced by exogenous physical agents, by endogenous chemical genotoxic agents that are the products of metabolism, such as reactive oxygen species (ROS), or by spontaneous chemical reactions, such as hydrolysis. Examples of DNA damage are ultraviolet (UV)-induced photoproducts (left), interstrand and intrastrand crosslinks, bulky chemical adducts (purple sphere), abasic sites, and oxidative damage such as 8-oxoguanine (8-oxoG). The consequences of DNA damage are essentially twofold. After misrepair or replication of the damaged template, surviving cells may be subject to permanent changes in the genetic code in the form of mutations or chromosomal aberrations, both of which increase the risk of cancer. Alternatively, damage may interfere with the vital process of transcription or induce replication arrest, which may trigger cell death or cellular senescence, contributing to aging. Damage-induced cell death protects the body from cancer. G denotes guanine, and T thymidine.
|
|
|
DNA Repair
|
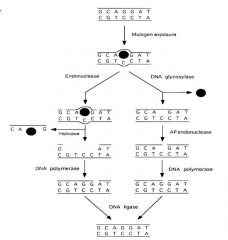
|
|
|
Describe liver functions
|
*he liver has several functions that are segregated into different regions of the organ.
* Bioactivation and detoxification: The liver transforms many compounds through a series of enzymatic reactions. * Respiration: Metabolized sugars and amino acids are processed through the Krebs cycle. * Synthesis & Storage: The liver acts as a storage depot for a number of different compounds. |
|
|
What is the most frequent reason cited for the withdrawal from the market of an approved drug?
|
Drug-Induced Hepatic Injury
|
|
|
What accounts for more than 50% of the cases of acute liver failure in the USA?
|
Drug-Induced Hepatic Injury
|
|
|
What do more than 75% of cases of idiosyncratic drug reactions result in?
|
liver transplantation or death
|
|
|
About 1000 drugs have been implicated in causing what?
|
liver disease
|
|
|
Describe liver dysfunctions
|
Dysfunctions in nutrient homeostasis
• Hypoglycemia, confusion • Hypercholesterolemia Dysfunctions in protein synthesis • Excess bleeding due to decreased clotting factors • Hypoalbuminemia, ascites • Fatty liver due to decreased transport proteins i.e. VLDL Dysfunction in the filtration of particles • Endotoxemia due to an increase in the products of intestinal bacteria such as endotoxin Dysfunction of biotransformation and detoxification processes • Jaundice, hyperammonemia-related coma due to bilirubin and ammonia increases • Loss of secondary male characteristics due to steroid hormones increase • Diminished drug metabolism due to xenobiotics (?) Dysfunction in the formation of bile and biliary excretion * Fatty diarrhea, malnutrition, vit E deficiency due to decreased bile acid-dependent uptake of dietary lipids and vits * Jaundice, hypercholesterolemia due to increase in bilirubin and cholesterol * Mn-induced neurotoxicity due to accumulation of metals such as Cu and Mn * Delayed drug clearance due to xenobiotics (?) |
|
|
Describe the six mechanism of liver toxicity
|
1) Disruption of intracellular calcium homeostasis leads to the disassembly of actin fibrils at the surface of the hepatocyte, resulting in blebbing of the cell membrane, rupture, and cell lysis.
2) In cholestatic diseases, disruption of actin filaments may occur next to the canaliculus, the specialized portion of the cell responsible for bile excretion. Loss of villous processes and the interruption of transport pumps such as multidrug-resistance–associated protein 3 (MRP3) prevent the excretion of bilirubin and other organic compounds. 3) Many hepatocellular reactions involve the heme-containing cytochrome P-450 system, generating high-energy reactions that can lead to the covalent binding of drug to enzyme, thus creating new, nonfunctioning adducts. 4) These enzyme–drug adducts migrate to the cell surface in vesicles to serve as target immunogens for cytolytic attack by T cells, stimulating a multifaceted immune response involving both cytolytic T cells and cytokines. 5) Activation of apoptotic pathways by tumor necrosis factor (TNF- ) receptor or Fas may trigger the cascade of intercellular caspases, which results in programmed cell death with loss of nuclear chromatin. 6) Certain drugs inhibit mitochondrial function by a dual effect on both - oxidation (affecting energy production by inhibition of the synthesis of nicotinamide adenine dinucleotide and flavin adenine dinucleotide, resulting in decreased ATP production) and the respiratory-chain enzymes. Free fatty acids cannot be metabolized, and the lack of aerobic respiration results in the accumulation of lactate and reactive oxygen species. The presence of reactive oxygen species may further disrupt mitochondrial DNA. This pattern of injury is characteristic of a variety of agents, including nucleoside reverse-transcriptase inhibitors, which bind directly to mitochondrial DNA, as well as valproic acid, tetracycline, and aspirin. Toxic metabolites excreted in bile may damage bile-duct epithelium (not shown). DD denotes death domain. |
|
|
What are idiosyncratic reactions?
|
Occur independently from dose. They are quite rare and occur rapidly after the initial dose and are severe with short duration.
Hypersensitivity (Acute cholestatic hepatitis after sulfasalazine administration, halothane). Genetic polymorphisms (Isoniazid). |
|
|
What are the predictable reactions (depend on what), know at least three examples from Table 2
|
Usually are dose dependent and occur in greater than 2% of the population taking the drug in question (Acetaminophen).
Examples: •APAP – increased dose: hepatocyte necrosis, apoptosis •Amiodarone – Cumulative dose: steatohepatitis •Bromfenac – Cumulative dose: hepatocyte necrosis •Cocaine, phencyclidine – increased dose: ischemic necrosis •Cyclophosphamide – increased dose: hepatocyte necrosis (worse with increasing aminotransferase levels) •Cyclosporin – increased dose: cholestatic injury •Methotrexate – increased or cumulative dose: hepatocyte necrosis, fibrogenesis •Naicin – Increased dose: ischemic necrosis •Oral contracepeptives – cumulative dose: associated with hepatic adenomas |
|
|
Discuss the interaction between acetaminophen and ethanol.
|
APAP-induced direct hepatotoxicity:
•is largely due to the covalent binding of NAPQI (a quinine amine) to cellular proteins • when glutathione is depleted major toxicity can occur • Mitochondrial dysfunction and oxidative stress leading to liver injury When ETOH is present: •NAPQI formation is diminished •CYP2E1 degrades slower, the half-life increases from 7 hours to 37 hours. • As long as ethanol remains in the body, there is competition between acetaminophen and ethanol for CYP2E1 •CYP2E1 is temporarily more upregulated • Once ethanol is removed, NAPQI formation is enhanced, resulting in increased hepatic injury in the 24 hours after the cessation of alcohol consumption. |
|
|
What is the mechanism of the alcohol-acetaminophen syndrome?
|
When ETOH is present:
•NAPQI formation is diminished •CYP2E1 degrades slower, the half-life increases from 7 hours to 37 hours. • As long as ethanol remains in the body, there is competition between acetaminophen and ethanol for CYP2E1 •CYP2E1 is temporarily more upregulated • Once ethanol is removed, NAPQI formation is enhanced, resulting in increased hepatic injury in the 24 hours after the cessation of alcohol consumption. |
|
|
What is fatty liver?
|
Steatosis (fatty liver): Increase of hepatic lipid content more than 5% by weight. Common response to acute exposure. Often reversible.
Major causes are: Alcohol Drugs Obesity |
|
|
Halothane (Liver)
|
Example of Combined Toxic and Allergic Reactions
Toxicity requires multiple exposures to halothane. Patients present with centrolobular necrosis in one of 2 ways: a. Elevation of serum transaminases with no external symptoms. This indicates an early stage which can be easily treated. b. Nausea, vomiting, upper abdominal pain, and jaundice. This represents a more advanced stage which is accompanied by significant liver damage. Without intensive care, this condition is fatal |
|
|
How does rifampicin contributes to acetaminophen toxicity (mechanism)?
|
Rifampicin induces CYP3A4
|
|
|
What is the mechanism of acetaminophen toxicity (slide 20)?
|
CYP2E1-mediated metabolic activation and oxidative stress following APAP treatment can cause irreversible inhibition of fatty acid oxidation, potentially through suppression of PPARα-regulated pathways
|
|
|
Define cholestasis and drugs that they cause it.
|
* Hepatic clearance of drugs depends on the activity of transport proteins that are located on the hepatocyte canalicular membrane.
* Alterations of these transporters by drugs or genetic polymorphisms increase the susceptibility to cholestatic injury. * Decrease in the volume of bile formed or impaired secretion of specific solutes into bile. * Elevated serum levels that are normally concentrated in the bile (bile acids and bilirubin). * Jaundice appears early. * Chlorpromazine induced hepatocellular cholestasis in approximately 1% of the patient population. It causes a precipitation of bile salts and thus decreases bile flow. The reaction usually begins in the first 4 months of treatment, but can take months to develop in some cases. * Other drugs, which have been reported to cause rare cases of cholestasis include: sulfonamides, sulfonylureas, erythromycin, and captopril. |
|
|
Cholestatic jaundice and estrogen therapy.
|
* This liver disease involves damage to the bile canaliculi and bile ducts.
* Long term estrogen therapy has been implicated in this disease. * The decrease in estrogen doses in oral contraceptives is proportional to an observed decrease in cholestatic jaundice. |
|
|
Bile duct damage (drugs).
|
* Known also as cholangiodestructive cholestasis.
* Increased serum levels of enzymes localized in the bile duct (alkaline phosphatase). |
|
|
The case of Vitamin E was discussed.
|
* Premature infants often have a vitamin E deficiency.
* Treatment with a-tocopherol acetate (an intravenous form of vitamin E) was associated with a huge incidence of cholestatic jaundice – approximately 10% of infants. * Within this population there was a 50% mortality rate. This caused the drug to be pulled from the market. |
|
|
What is cirrhosis and what kind of drugs cause cirrhosis?
|
* The end stage of chronic liver injury in which extensive amounts of fibrous tissue, especially collagen fibers are deposited in response to direct injury or inflammation.
* Methotrexate is used in patient with severe psoriasis and rheumatoid arthritis. * Toxicity may develop over a period of several years without any symptoms. * It causes fibrous deposits to some extent in all patients. * Bioactivation via P450 enzyme is the cause. This is most often seen in patients being treated for psoriasis. * Damage can be controlled by decreasing the dose (or increasing the interval between doses). * Vitamin A, normally stored in the space of Disse, can cause significant fibrosis if taken for extended periods of time. * Methyldopa. * Alcohol. |
|
|
Allergic Hepatitis (all drugs they are induced it)?
|
* Liver-specific inflammatory reaction caused by hypersensitivity to a particular drug.
* Bioactivation is often required for hepatitis to occur but not through the production of toxic metabolites. Instead, these metabolites can function as haptens to promote an immune response to cellular proteins. * Less common than other forms of drug-induced hepatotoxicity, but has more serious clinical implications. * Implication of the immune system in the pathogenesis of many drug hypersensitivity reactions. * The onset of a hypersensitivity reaction involves covalent binding of the drug to proteins to form immunogenic conjugates. * Clinically, both hepatocellular injury and cholestasis can occur, and most episodes have good clinical prognoses upon drug withdraw. |
|
|
Drug-induced chronic hepatitis (drugs).
|
Drug-Induced Chronic Hepatitis-(Methyldopa)
* Indolent liver damage that closely resembles autoimmune chronic hepatitis. * Early identification of drug-induced chronic hepatitis is not easy. * Identification of the drug causing this liver damage might is difficult retrospectively. * In addition to methyldopa, acetaminophen, aspirin (in high doses), nitrofurantoin, trazodone, ketoconazole, halothane have been determined to cause this syndrome. * Multiple prescription renewals may be a problem in the of nitrofurantoin, which is used to control recurrent urinary tract infections. |
|
|
Microvesicular steatosis (drugs)
|
* Microvesicular steatosis is characterized by an abnormal accumulation of numerous small cytoplasmic lipid droplets in hepatocytes.
* Fulminant or progressive cases of microvesicular steatosis may lead to liver failure and death. * Fine vesicles are associated with cellular dysfunction but without cell death. * This is characteristic lesion of fatty liver caused by pregnancy, tetracyclines, and the Reye’s syndrome associated with aspirin. * Death due to steatosis has been observed in pregnant women after the intake of tetracyclines. * The more modern tetracyclines, minocycline and doxycycline are rarely, if ever, associated with these symptoms. * Mechanism: Inhibition of the b-oxidation of fatty acids. * Macrovesicular steatosis occur in association with AIDS and the use of zidovudine. * Tragic outcome with the fialuridine, a nucleoside analog used to treat hepatitis B. * Severe or fatal lactic acidosis associated with macrovesicular steatosis after eight weeks of therapy. |
|
|
What is the rye’s syndrome?
|
* An aggressive form of liver disease associated with aspirin when administered to children who suffer from viral infection.
* The mitochondria are damaged leading to depletion of acetyl-CoA and carnitine. * Fatty acids accumulate and gluconeogenesis is impaired, leading to hypoglycemia. * A rapid form of advanced steatonecrosis ensues, which is often fatal. |
|
|
FDA’s MedWatch Program
|
* Pharmaceutical companies are required to report any serious adverse events to the agency within 24 hrs.
* Surveillance becomes a passive process once the drug is in the market. * Physicians and pharmacists may voluntarily file written reports. * It is estimated that MedWatch receives fewer than 10% of ADR reports. |
|
|
Drugs recently withdrawn from the market (know the drugs and toxicity).
|
* Bromfenac, a NSAID was introduced in 1997 as a short term analgesic for orthopedic pain.
* The FDA approved the drug for periods of 10 days or less. * Once released, the drug was associated with more than 50 cases of severe liver injury, and the drug was withdrawn in June 1998. * All patients with liver toxicity had taken the drug for more than 30 days. * Troglitazone (Rezulin) a PPARg antagonist which reduces insulin resistance and increases insulin-stimulated glucose disposal. * In clinical trials, reversible elevated levels of SAT were observed, occasionally exceeding 8 times the upper physiologic limit. * Once the drug was approved reports of severe and fatal liver injury began to appear. * After a total of 90 cases (68 fatal and 10 necessitated transplantation the drug was withdrawn. * Lumiracoxib COX-2 selective inhibitor. * Pemoline (Cylert) is a medication used to treat attention-deficit hyperactivity disorder (ADHD). * Ximelagatran (Exanta or Exarta) is an anticoagulant that has been investigated extensively as a replacement for warfarin. * Trovafloxacin (Trovan, Turvel) is a broad spectrum antibiotic that inhibits the uncoiling of supercoiled DNA in bacteria by blocking the activity of DNA gyrase and topoisomerase IV. |
|
|
What is the problem in predicting idiosyncratic reactions?
|
* Occur independently from dose. They are quite rare and occur rapidly after the initial dose and are severe with short duration.
* Death can occur in as little as 1 hour * Hypersensitivity (Acute cholestatic hepatitis after sulfasalazine administration, halothane). * Genetic polymorphisms (Isoniazid). * The classic example is isoniazid. * 15-20% of the patients have elevated plasma alanine and aspartate aminotransferase levels. * Only 1% will develop hepatic necrosis that will require the withdrawal of the drug. * The role of genetic polymorphisms in NAT gene. |
|
|
Why we have kidney toxicity?
|
* Toxic nephropathy is an important cause of reversible renal injury if detected early.
* Toxic nephropathy is a general term which describes renal damage caused by either exogenous or endogenous compounds leading to renal dysfunction. * Renal damage can be due to several different mechanisms affecting different segments of the nephron, renal microvasculature or interstitium. |
|
|
Details on cyclosporin A in renal toxicity
|
* It is an important immunosuppressive agent that is widely used to prevent graft rejection in organ transplantation.
* It selectively inhibits T-cell activation. * Nearly all patients who receive the drug exhibit some form of nephrotoxicity. (Death can result.) * Dose-dependent induction of renal vasoconstriction. |
|
|
What drugs cause pre-renal toxicity?
|
NSAIDs, ACE inhibitors, and Cyclosporin A
|
|
|
Which conditions or disease are causing stressed kidney?
|
Hypovolaemia, cardiac failure, pre-existing renal disease, hepatic dysfunction, renal vascular disease and salt depletion, patients in diuretic or cyclosporin therapy, and elderly.
|
|
|
Details on ACE inhibitors in reanal toxicity
|
* With renal stenosis, GFR is only maintained by a stimulated renal-angiotensin system. This increases resistance at the efferent arteriole.
* Predictably, the use of ACE inhibitors interferes with this compensation and also compounded by the desired effect of lowered blood pressure. * Decreases in GFR can therefore be severe. Deterioration in function is usually reversible. |
|
|
Acute interstitial nephritis (know the drugs causing it.)
|
Acute Interstitial Nephritis - Monocellular cell infiltrate with edema in the renal interstitium.
Drugs associated with interstitial nephritis: * Antibiotics: Methicillin, sulphonamides, rifampicin. * Diuretics: Thiazides, furosemide. * Miscellaneous: Phenytoin, cimetidine, mefenamic acid. * NSAIDs |
|
|
Acute tubular necrosis (details on drug causing ATN).
|
- Aminoglycoside antibiotics
- Amphoterecin B - Acetaminophen - Cisplatin - Tetracycline - Cephalosporins - Cyclosporin A |
|
|
Obstruction (drugs).
|
Amphoteresin B, Methotrexate, and Acyclovir
|
|
|
Chronic renal failure (role of NSAIDS).
|
* The most common cause of drug-induced failure is analgesic nephropathy manifested as papillary necrosis and interstitial nephritis.
* Also have impaired renal-concentrating capacity. * Continued use of analgesics results in end-stage renal disease. * The precise mechanism of analgesic nephropathy is unclear but probably arises from some combination of medullar ischemia. |
|
|
Age dependent changes in the kidneys (importance, reason, and consequences).
|
* A frequent cause of acute renal failure in the elderly is drug-induced nephropathy.
* NSAIDs, antibiotics, and diuretics are most often involved. Due to the age-dependent decline of renal function, the pharmacokinetics of many drugs are altered in elderly patients. * Declining renal function, a reduction in both RBF and GFR, is a major contributor to drug toxicity in the elderly. * Therefore, the most important renal function to monitor with aging is the creatinine clearance. |
|
|
Types of Drug Related Problems
|
Improper drug
Sub-therapeutic dose Drug interaction ADR Overdose Untreated |

