![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
69 Cards in this Set
- Front
- Back
|
How many barriers separate separate the ICF, interstitial fluid and plasma
|
2
|
|
|
what is the function of plasma membrane
|
separates ICF from surrounding interstitial fluid
|
|
|
what is the functional of blood vessels
|
to divide interstitial fluid from plasma
|
|
|
when is the body in fluid balance
|
when required amounts of waterand solutes are present and correctly proportioned among compartments
|
|
|
what is the largest component in the body making up 45-75% of total body mass
|
Water
|
|
|
what dos these processes allow:filtration reabsorption, diffusion, and osmosis
|
continual exchange of water and solutes among compartments
|
|
|
how is body water volume regulated
|
--How much we drink ( controlled by hypo)
|
|
|
how much percentage drop in water results in dehydration
|
2%
|
|
|
what stimulates the thirst centre in the hypothalamus
|
dehydration stimulates RAA pathway which stimulates thirst centre.
it is also stimulated by neurones in the mouth and baroreceptors |
|
|
how much water is ingested in liquids
|
1600ml
|
|
|
how much water is ingested through food
|
700ml
|
|
|
how much water is obtain metabolically
|
200ml
|
|
|
in terms of water movement, what does increasing osmolarity of interstitial fluiddo ?
|
it draws out water out of cells and makes cells shrink
|
|
|
Normally, cells neither shrink or swell because____________
|
intracellular and interstitial fluids have the same osmolarity
|
|
|
what does decreasing osmolarity do
|
makes cells swell
|
|
|
changes in osmolarity most often result from.....
|
changes in Na+ concentration
|
|
|
what is water intoxication ?
|
drinking water faster than the kidneys need it
|
|
|
what does water intoxication lead to ?
|
convulsions, coma or death
|
|
|
what are the functions of aldesterone ?
|
regulation of blood volume
blood pressure levels on Na+ , K+, H+ in the blood |
|
|
what causes loss of excess water an solute
|
urinary salt loss
|
|
|
what are the 2 main solutes in ECF
|
Na+ and Cl+
|
|
|
what is a solute
|
substance dissolved in a solution
|
|
|
what are the 3 most important hormones for regulating ion concentrations in the body
|
angiotensin II, aldosetrone and ANP
|
|
|
Hormonal regulation of renal Na+ and Cl- reabsorption
|
Increased intake of NaCl leads to increased plasma concentration of Na+ and Cl- which leads to increased osmosis of water from intracellular fluid to interstitial fluid to plasma and then leads to increased blood volume
|
|
|
what are 2 things increased blood volume leads to in the regulation of Na+ and Cl-
|
increased strectching of atria of the heart or decreased release of renin by juxtaglomerular cells
|
|
|
ADH
|
– Major hormone regulating water loss is
antidiuretic hormone (ADH) – Also known as vasopressin – Produced by hypothalamus, released from posterior pituitary – Promotes insertion of aquaporin‐2 into principal cells of collecting duct – Permeability to water increases, produces concentrated urine – Also stimulated by severe blood loss |
|
|
Electrolytes in body fluids
|
Ions form when electrolytes dissolve and dissociate
• 4 general functions – Control osmosis of water between body fluid compartments – Help maintain the acid‐base balance – Carry electrical current – Serve as cofactors |
|
|
most common ions in ecfs
|
Na+ Cl- HCO-3
|
|
|
most common ions in ICF
|
K, HPO2 protein anions
|
|
|
Sodium Na+
|
Sodium Na+
– Most abundant ion in ECF – 90% of extracellular cations – Plays pivotal role in fluid and electrolyte balance because it accounts for almost half of the osmolarity of ECF – Level in blood controlled by • Aldosterone – increases renal reabsorption • ADH – if sodium too low, ADH release stops • Atrial natriuretic peptide – increases renal excretion |
|
|
how adh conserves water
|
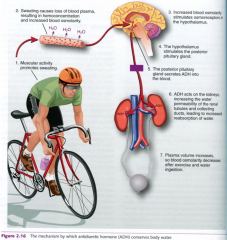
|
|
|
INFLUENCE of water loss from plasma...
|
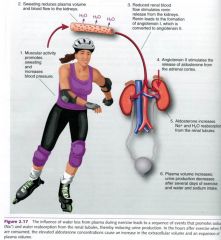
|
|
|
Chloride Cl‐
|
– Most prevalent anions in ECF
– Moves relatively easily between ECF and ICF because most plasma membranes contain Cl leakage channels and antiporters – Can help balance levels of anions in different fluids • Chloride shift in RBCs – Regulated by • ADH – governs extent of water loss in urine • Processes that increase or decrease renal reabsorption of Na+ also affect reabsorption of Cl |
|
|
Potassium K+
|
– Most abundant cations in ICF
– Key role in establishing resting membrane potential in neurons and muscle fibres – Also helps maintain normal ICF fluid volume – Helps regulate pH of body fluids when exchanged for H+ – Controlled by aldosterone – stimulates principal cells in renal collecting ducts to secrete excess K+ – Hyperkalemia can cause death by ventricular fibrilation |
|
|
Calcium Ca2+
|
– Most abundant mineral in body, most in skeleton and teeth
– Plays important roles in blood clotting, neurotransmitter release, muscle tone, and excitability of nervous and muscle tissue |
|
|
what hormone regulates ca2+
|
parathyroid hormone
|
|
|
how does parathyroid hormone regulate ca2+
|
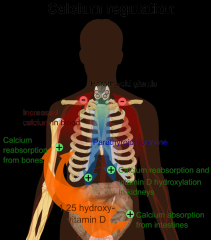
Stimulates osteoclasts to release calcium from bone ECM
Also enhances reabsorption from glomerular filtrate |
|
|
Magnesium
|
– In adults, about 54% of total body magnesium is part of
bone as magnesium salts – Remaining 46% as Mg2+ in ICF (45%) or ECF (1%) – Second most common intracellular cation – Cofactor for certain enzymes and sodium‐potassium pump – Essential for normal neuromuscular activity, synaptic transmission, and myocardial function – Secretion of parathyroid hormone depends on Mg2+ – Regulated in blood plasma by varying rate excreted in urine |
|
|
Phosphate
|
– About 85% in adults present as calcium phosphate salts in bone
and teeth – Remaining 15% ionized – H2PO4- , HPO42‐ , and PO43‐ are important intracellular anions – H2PO4- important buffer of H+ in body fluids and urine – Same hormones governing calcium homeostasis also regulate HPO4 2‐ in blood • Parathyroid hormone – stimulates resorption of bone by osteoclasts releasing calcium and phosphate, but inhibits reabsorption of phosphate ions in kidneys (PTH increases urinary excretion & lowers plasma levels) • Calcitrol promotes absorption of phosphates and calcium from GI tract |
|
|
what is hypernatremia
|
implies a plasma sodium level above 145 mEq/L.
|
|
|
causes of hypernatremia
|
dehydation, thirst from exercise and NaCl administration
|
|
|
consequences of hypernatremia
|
thirst. confusion, lethergy progressing to coma. increased neuromuscular irritability evidenced by twitching and convulsions
|
|
|
Hyponatremia
|
represents a plasma sodium concentration below 135mEq/L
|
|
|
causes of hyponatremia
|
solute loss, water rentention or both e.t through vommiting and diarhea burned skin tubal drainage of stomach/
|
|
|
consequences of hyponatraemia
|
neurologic dysfunction due to brain swelling. mental confusion giddiness and coma musclular twitching, irritability due to excess. if accompanied by water loss it leads tolow bp and low blood volume
|
|
|
Potassium level of 5.5 or higher is termed?
|
hyperkalemia
|
|
|
causes of hyperkaleamia
|
renal failure, deficit of aldesterone, rapid intravenoys infusion, burns or severe tissues and injuries which cause K to leave cells
|
|
|
consequences of hyperkaleamia
|
nausea vomitting, diarhea, depression, skeletal muscle weakness
|
|
|
hypokaleami
|
levels of potassium lower tham 3.5mEq/L
|
|
|
causes of hypokaleamia
|
gastrountestinal tract disturbances, gastrointestinal suction
|
|
|
consequences of hypokalaemia
|
flattened T wave, muscular weakness, metabolic alkalosis, mental confusion, nausea, vomiting
|
|
|
Hypocalcemia
|
<8.5 mg/dL or an ionized calcium level of <4.0 mg/dL
|
|
|
Hypercalcemia
|
>10.5 mg/dL or an ionized calcium level of >5.0 mg/dL ; caused by malignancy or prolonged immobilization
|
|
|
Hypomagnesemia
|
<1.5 mEq/L ; chronic alcholism is the most common cause
|
|
|
Hypermagnesemia
|
>2.5 mEq/L ; due to increas intake or decrease excretion
|
|
|
Hypochloremia
|
<95 mEq/L
|
|
|
Hyperchloremia
|
>108 mEq/L ; excess replacement of sodium chloride or potassium chloride
|
|
|
Hypophosphatemia
|
<2.5 mg/dL; glucose and insulin administration, parenteral nutrition(feeding through IV)
|
|
|
Hyperphosphatemia
|
>4.5 mg/dL ; tissue trauma, chemotherapy, renal failure
|
|
|
causes of hypercalcemia
|
malignancy and hyperparathyroidism,bone loss related to immobility
|
|
|
causes of hypocalcemia
|
causes of hypocalcemia
|
|
|
Respiratory acidosis
|
can be caused by emphysema, pulmonary edema, injury to the respiratory centre of the medulla, airway obstruction etc
The kidneys can compensate if mild Treatment include ventilation therapy and IV of HCO3- |
|
|
Respiratory alkalosis
|
caused by hyperventilation due to overstimulation of inspiratory centre.
Kidneys can compensate if mild, a paper bag can help |
|
|
Metabolic acidosis
|
caused by diarrhoea, renal dysfunction, acid accumulation (e.g. ketosis)
Hyperventilation can compensate if mild Treatment include IV of sodium bicarbonate solution |
|
|
Metabolic alkalosis
|
caused by loss of acid (mainly vomiting) or excessive intake of alkaline drugs eg diuretics or antacids
Hypoventilation can compensate if mild, need to replace electrolytes |
|
|
Kidney excretion of H+
|
– Metabolic reactions produce non-volatile acids
– One way to eliminate this huge load is to excrete H+ in urine – In the proximal convoluted tubule, Na+ /H+ antiporters secrete H+ as they reabsorb Na+ – Intercalated cells of collecting duct include proton pumps that secrete H+ into tubule fluid – Urine can be up to 1000 times more acidic than blood – 2 other buffers can combine with H+ in collecting duct • HPO42‐ and NH3 |
|
|
Acid‐base balance
|
• Major homeostatic challenge is keeping H+ concentration (pH) of body fluids at appropriate level
• 3D shape of proteins sensitive to pH • Diets with large amounts of proteins produce more acids than bases which acidifies blood • Several mechanisms help maintain pH of arterial blood between 7.35 and 7.45 – Buffer systems, exhalation of CO2, and kidney excretion of H+ |
|
|
– Phosphate buffer system
|
• Dihydrogen phosphate (H2PO4‐) and monohydrogen phosphate(HPO42‐ )
• Phosphates are major anions in ICF and minor ones in ECF • Important regulator of pH in cytosol |
|
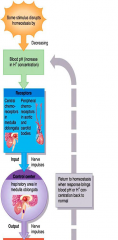
exhalation of CO2
|
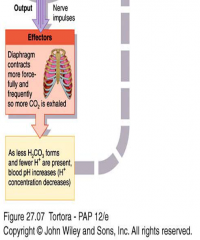
|

