![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
28 Cards in this Set
- Front
- Back
|
Define ionic bonding |
The bond that forms as a result of the attraction between positively and negatively charged ions |
|
|
Ionic compounds |
Compounds that are composed of positive ions and negative ions |
|
|
MgCl2 is |
Magnesium chlorine |
|
|
MgCl2 Lewis diagram |
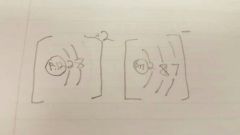
|
|
|
What is the formula for aluminium plus sulfur |
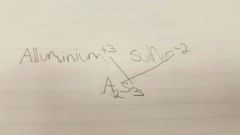
|
|
|
Mg3(PO4)2 |
Magnesium sulphate |
|
|
Sodium nitrate |
Na(NO3)2 |
|
|
Calcium hydroxide |
Ca(OH)2 |
|
|
Ammonium sulphide |
(NH4)2 S |
|
|
FeCl3 |
Iron III Chlorine |
|
|
MnSO3 |
Manganese II Sulphite |
|
|
Nickel III Chloride |
NiCl3 |
|
|
Vanadium (IV) sulphate |
V2 (SO4)4 |
|
|
Iron (III) phosphide |
Fe3P3 |
|
|
Define Covalent bonding |
The formation of a chemical bond between atoms through the sharing of 1 or more electrons |
|
|
Define Covalent compounds |
A compound formed when non metalic atoms share electrons to form Covalent bonds |
|
|
Define Bonding pair |
A pair of electrons involved in a Covalent bond |
|
|
Lone pair |
A pair of electrons in an atoms valence shell that I'd not used in bonding |
|
|
N2O |
Nitrogen dioxide |
|
|
PI3 |
Phosphorous triodide |
|
|
S2F10 |
Disulphur decaoxide |
|
|
O2 |
Dioxide |
|
|
Lewis diagram PI3 |
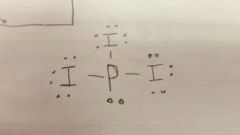
|
|
|
Lewis diagram O2 |
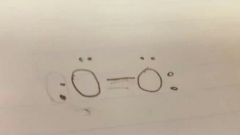
|
|
|
Sulphur diflouride |
SF2 |
|
|
Diiodide hexachloride |
I2Cl6 |
|
|
Phosphorous pentaflouride |
PF5 |
|
|
Lewis diagram Sulphur diflouride |
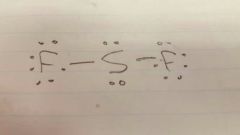
|

