![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
22 Cards in this Set
- Front
- Back
|
hydrochloric acid
|
HCl: strong acid
|
|
|
Nitric acid
|
HNO3: strong acid
|
|
|
Sulfuric acid
|
H2SO4: Strong acid
|
|
|
Hydrobromic acid
|
HBr: strong acid
|
|
|
Hydroiodic acid
|
HI: strong acid
|
|
|
Perchloric acid
|
HClO4: strong acid
|
|
|
OH complexes
|
strong base
1)Alkali metal hydroxide complexes example: NaOH 2)Alkaline earth metal hydroxide complexes: Ca(OH)2 |
|
|
Formic acid
|
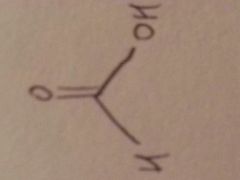
HCOOH: weak acid
|
|
|
Acetic acid
|
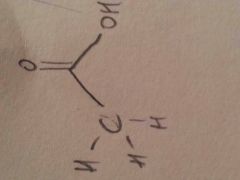
CH3COOH: weak acid
|
|
|
Trichloroacetic acid
|
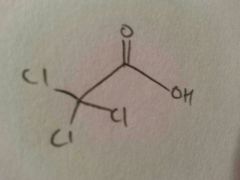
CCl3COOH: weak acid
|
|
|
Hydrofluoric acid
|
HF: weak acid
|
|
|
Hydrocyanic acid
|
HCN: weak acid
|
|
|
hydrogen sulfide
|
H2S: weak acid
|
|
|
water
|
H2O: weak acid/ weak base
|
|
|
ammonia
|
NH3: weak base
|
|
|
Trimethyl ammonia
|
N(CH3)3: weak base
|
|
|
pyridine
|
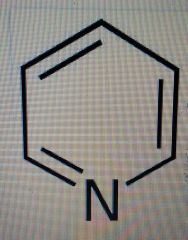
C5H5N: weak base
|
|
|
Ammonium hydroxide
|
NH4OH: weak base
|
|
|
Hydrogen sulfide ion
|
HS- :weak base
|
|
|
Cu(OH)2
|
weak base: (Cu2+ is much stronger than water and will undergo hydrolysis
|
|
|
metal hydrides, NaH, KH
|
strong base (lewis)
NaH + H2O → H2 + NaOH |
|
|
butyl Lithium and other organic-lithium compounds
|
strong base
|

