![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
44 Cards in this Set
- Front
- Back
|
Normal function of the colon |
–Convertliquid small intestinal contents to solid faeces –Reabsorptionof water and soluble salts –Secretemucinto lubricate motion |
|
|
Normal Structure of Colon |
–Epitheliumarranged in glands (crypts) --> Columnarabsorptive cells --> Mucousgoblet cells --> Stemcells –Laminapropria –Muscularismucosa –Muscularisexterna –Subserosa –Serosa |
|
|
typical lifespan of colonic cells? |
- continually being replaced by stem cells (base of crypt) - they mature and rise to the lumen - dies and sloughs off after 4 days (via apoptosis) |
|
|
how common in CRC? |
- 2nd most common malignancy and cause of cancer related death - 1:12Australians will get bowel cancer - 1:25Australians will die from bowel cancer |
|
|
Who does CRC effect? |
- a disease of the elderly, slightly more common in men (peaks at 60-70 yo) 1 in 17 males 1 in 26 females (risk age 75yo) |
|
|
where is CRC most incidental |
Western disease where Aust, NZ have some of the highest numbers in the world along with USA, Canada, Denmark, Sweden Africa has low incidence |
|
|
Etiological factors associated with CRC |
DIET- assoc with low fibre diet, high refined carbohydrates, and fat (incidence reduced by dietary alterations?) GENETICS (20% Familial)- small percentage of bowel cancer is due to high penetrance hereditary conditions --> Familial Adenomatous Polyposis (<1%) --> Hereditary Non-polyposis coli (HNPCC) (~2%) Associated with IBD- UC, CD |
|
|
2 pathways of CRC pathogenesis |
1. Adenoma-carcinoma sequence 2. Multistep theory of carcinogenesis |
|
|
Polyps |
- Lesionwhich stands out above the surrounding mucosa (raised lesions) - Inflammatory,hyperplastic, haemartomatous, neoplastic Benignneoplasticpolyp=ADENOMA (Smallrisk that an adenoma may become invasive-> adenocarcinoma_ |
|
|
2 types of macroscopic polyps |
Sessile: seated, flat against lumen Pedunculated: stalk |
|
|
Architecture of adenomatous polyps |
- Tubular-small,rounded, tubular glands - Villous-longslender villi (look like cauliflowers) - Tubulovillous-mixed - Sessileserrated adenoma |
|
|
colonic adenoma prevalence |
- 20-30% before age 40 - 40-50%before age 60 - M=F - Familialpredisposition for sporadic adenomas |
|
|
Histology of Adenomas |
–Epithelial cells fail to mature as cells migrate out of crypt –Nuclear hyperchromasia –Stratification,crowding –Less mucin –Larger nuclei |
|
|
how do adenomatous polyps become invasive (malignant)? |
--> Relates to size (unlikely if <10mm, but >4cm- 40% contain foci of cancer) --> degree of dysplasia (severe= carcinoma in situ) |
|
|
how long does an adenoma take to develop, and it to become malignant? |
- Takes5-20 years to form adenoma - Takes5-15 years for an adenoma to become invasive |
|
|
What does the multi-step theory comprise of? |
- accumulation of mutations, epigenetic changes over time - TSG- regulate cell cycle, switch off (2 hits), (eg. APC, p53) -Protooncogenes- promote cell cycle, switch on (1 hit), (eg. kras) (Hyperplasia)->(Metaplasia)->Dysplasia/Benignneoplasia->Malignantneoplasia |
|
|
What does the Adenoma-Carcinoma sequence comprise of? |
Normal Colon--> inherited and acquired mutations of cancer suppressor genes "first hit" (APC at 5q21) Mucosa at risk--> methylation abnormalities inactivates normal alleles "second hit" (APC at b-catenin) Adenoma--> 1. proto-oncogene mutation (k-ras at 12p12) 2. loss of additional cancer suppressor genes and over expression of COX2 (p53, LOH, SMAD 1 and 2) Carcinoma--> additional mutations, gross chromosomal alterations (telomerase, many other genes) n |
|
|
Histological features of CRC |
- less mucin - larger nuclei - crowding- stratification -loss of polarity - nuclear and cellular pleomorphism -prominent nucleoli |
|
|
Clinical features of left sided CRC |
–Constrictioncausing altered bowel habit –Brightblood per rectum –Annularlesion (napkin ring) –Changesin bowel habit-constpation/diarrhoea–Tenesmus -feeling of incomplete evacuation (rectal) |
|
|
Clinical features of right sided CRC |
–Occultbleeding causing anaemia –Polypoidexophyticmass –Rarelycause obstruction –Fatigue,weakness, iron def anemia |
|
|
General clinical manifestations of CRC |
- Irondef anaemia inolder man or post- menopausal woman=GI cancer until proven otherwise - Bleedingmixed with stool-urgent investigation - - Maybe assoc with mucus - Abdominalpain-cramping, gnawing, - Obstruction- colicky lower abdominal pain - Perforation-localizedor generalized pain, back pain ifretroperitoneal |
|
|
diagnostic tests available for CRC? |
- Faecal Occult Blood Test (FOBT) - Colonoscopy and biopsy - CT scan of abdomen (staging- mets?) - Virtual Colonscopy (?) |
|
|
What can we do with macroscopic and microscopic CRC specimens? |
- Macroscopic --> Patternsof growth - Microscopic --> Architecturalpatterns --> Tumourgrade (differentiation) |
|
|
How can CRC spread? |
Local invasion - extension through bowel wall Metastatic spread - lymphatic spread - haematogenous spread to liver, lung, bones and beyond - transcavity spread |
|
|
2 most important prognostic factors in staging CRC? |
–Depthof invasion (invasion into muscularispropria-reducedsurvival) –Lymphnode mets (TNM) |
|
|
TNM criteria |
T1: Tumourinvades the submucosa T2: Tumourinvades into but NOT beyond the musc. propria T3: Tumourinvades into subserosa butnot serosa (iethrough musc propria) T4: Tumourinvades into serosa orother organs N1:1-3 regional lymph nodes N2:>3 regional lymph nodes M1:Distant metastasis |
|
|
Staging tumour based on TNM |
StageI T1or T2, no nodes (N0), no mets (M0) StageII T3 or T4, no nodes (N0), no mets (M0) StageIII Any T, nodes pos (N1 or 2), no mets (M0) StageIV Any T, Any N, distant mets (M1) |
|
|
Treatment options for CRC |
- Surgery (segmental resection) - Chemo (adjuvant Stage III, palliative Stage IV) -Radiotherapy (neoadjuvant for rectal tumours) |
|
|
Tumour Stage and Survival rates |
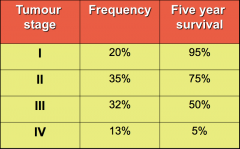
|
|
|
the 2 models of colorectal carcinogenesis |
- Theclassical pathway --> chromosomal instability and the lessons from FAP (Familial Adenomatous Polyposis) - Themismatch repair pathway --> microsatellite instability and the lessons from Lynch Syndrome |
|
|
Chromosomal instability in FAP (familial adenomatous polyposis) |
- FAP is autosomal dominant in 1% of all CRC - caused by APC gene mutation on 5q - if colon is not removed, inevitable CRC by age 40 kras mutation in early adenoma --> TP53 mutation in adenoma to cancer transitions |
|
|
what kind of diseases can FAP cause? |
causes duodenal adenomas, desmoid tumours, osteomas, thyroid cancer, brain tumour ETC |
|
|
what is APC's function? |
NORMALLY - regulates the upward migration of cells from base to crypt - regulates microtubules that form mitotic spindles WHEN MUTATED: - fails to migrate cells upwards and accumulates --> may generate polyps - cells increase in chromosomal instability |
|
|
What is the function of TP53? (POLICING DNA INTEGRITY) |
NORMALLY: - cells containing p53, detects DNA damage which activated p53 and bind to DNA for successful repair (if unsuccessful- apoptosis) WHEN MUTATED: - detection of DNA damage, doesn't activates p53 dependant genes - there is no cell cycle arrest or DNA repair - mutant cells are formed and expansion of additional mutations occur = malignant tumour |
|
|
what is the microsatellite pathway? |
- Characterised bymicro satellite instability - Dueto loss of mismatch repair enzymes - BRAFmutationstypical - APC, KRAS and TP53changes rare (no micro satellites in them) - Keyrole of promoter methylation in sporadic disease (tends to effect women more) |
|
|
what are the 4 mismatch repair (MMR) genes? |
- MLH1(mainly in sporadic) - MSH2 (more common in lynch syndrome) - MSH6 - PMS2 |
|
|
What is a germ line mutation? |
inherited mutation |
|
|
What is a sporadic mutation? |
acquired mutation |
|
|
genetic features of Lynch syndrome? |
-autosomal dominant inheritance - penetrance for CRC 80% - caused by germ line mutations in any of the 5 genes in DNA MMR family |
|
|
How to diagnose lynch syndrome? |
- Family history - Tumour (pathology, MSI, MMRD) - Detect germline mutations in MMR |
|
|
the histology of (macro and micro) of lynch syndrome (quite distinctive)? |
- mostly right sided tumours (proximal to splenic flexure) - mucinous, poorly differentiated - intraepithelial lymphocytes common - loss of MSH2 and MLH1 staining |
|
|
how to detect MSH2 and MLH1 in IHC? |
- Lossof protein expression by IHC correlates very well with MSI status - Lossof MSH2 = germ linemutation - Lossof MLH1 expression also seen in sporadic (non-hereditary cases) |
|
|
how are sessile serrated adenomas characterised on H&E staining slide? |
- basal dilation of the crypts - serration of bases -branching in crypts |
|
|
Summary of CRC development pathway |
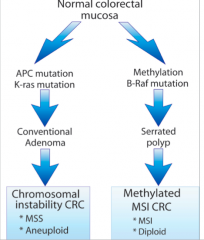
|

