![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
155 Cards in this Set
- Front
- Back
|
Ways H2O and electrolytes can enter plasma:
|
-alimentary tract
-cells -injections and infusions |
|
|
How might Potassium be added to plasma or serum?
|
-in vitro lysis of RBCs
-platelet activation |
|
|
Ways H2O and electrolytes may leave the plasma and the body:
|
-kidneys
-alimentary tract -respiratory tract -skin -extravascular sites (3rd space loss) |
|
|
What electrolyte changes may occur in pairs?
|
-inc. Na & inc. Cl
-inc. Cl & dec. HCO3 |
|
|
Plasma [Na] is nearly equivalent to:
|
ECF [Na]
|
|
|
ECF [Na] is dependent on:
|
-ratio of tbNa : tbH2O
|
|
|
Serum [Na] is controlled by:
|
-regulation of blood volume
-regulation of plasma osmolality |
|
|
Hypovolemia effects on serum [Na]
|
attempt to restore blood volume
-inc. RAS (inc. Na retention) -inc. ADH (inc. H2O retention) -dec. ANP (dec. Na excretion) |
|
|
Increased Osmolality effects on serum [Na]
|
attemp to dilute solutes
-inc. ADH (inc. H2O retention) -thirst centers (inc. H2O intake) |
|
|
Normonatremia ratio:
|
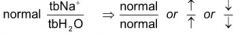
|
|
|
Hypernatremia ratio:
|
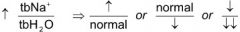
|
|
|
Hyponatremia ratio:
|
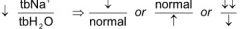
|
|
|
Pathologic dehydration
-definition |
-dec. in total body H20 characterized by either H2O loss or Na & H2O loss
|
|
|
Normonatremic (Isotonic) dehydration effect on [Na]
|
-lose isotonic fluid (H2O loss proportional to Na loss)
-[Na] in remaining fluid is the same but the animal is hypovolemic |
|
|
Effect of drinking water after isotonic fluid loss
|
-become hypotonic due to the dilution of electrolytes by the water
|
|
|
Effect of losing pure water from the vasculature
|
-become hypertonic due to increased electrolyte conc.
|
|
|
Ways pure water can be lost
|
-urine with a SG near 1.000
-water vapor in respiration |
|
|
Fluid losses from blood can be either:
|
-hypotonic
-isotonic -pure water |
|
|
Most common cause for hypernatremia
|
-dec. tb H2O (dehydration)
|
|
|
Reasons for dec. tb H2O
|
-dec. H2O intake
-inc. pure H2O loss -H2O loss > Na loss |
|
|
Reasons for dec. H2O intake
|
-restricted access to water
-defective thirst (occurs with continued daily loss of water) |
|
|
Reasons for inc. loss of pure H2O
|
-water vapor from hyperventilation, panting, fever (sweating)
-Diabetes insipidus |
|
|
Reasons for H2O loss > Na loss
|
-osmotic diuresis (glucose in tubular fluid)
-osmotic diarrhea (osmotic agents in alimentary system) |
|
|
Causes of hyponatremia
|
-H2O retention > Na retention (edematous state)
-Na loss > H2O loss (dehydration state) |
|
|
Reasons for H2O retention > Na retention causing edema or transudates
|
-heart failure
-cirrhosis -nephrotic syndrome |
|
|
Reasons for Na loss > H2O loss causing hyponatremia
|
Increased renal loss of Na
-hypoadrenocorticism -obligated loss with anions -diuretics -renal disease -osmotic diuresis (if concurrently drinking water) |
|
|
Hypoadrenocorticism (addison's disease) causing hyponatremia
-pathogenesis |
-dec. aldosterone
-dec. renal conservation of Na -dec. tb Na -dec. cortisol -dec. inhibition of ADH release -inc. cortisol -inc. H2O retention -dilute remaining Na -hyponatremia |
|
|
Conditions that will cause hyponatremia due to obligate Na loss with anions
|
-ketosis (AcAc, beta-hydroxybutyrate)
-metabolic alkalosis (HCO3-) -Hypoxia (lactate) |
|
|
Inc. intestinal loss of Na causing hyponatremia
-pathogenesis |
-Diarrhea and/or vomiting
-sequestration of fluid in the intestine (horse) -lose Na and H2O -dehydration -drink H2O -renal retention of H2O -hyponatremia |
|
|
Inc. cutaneous loss of Na causing hyponatremia
-pathogenesis |
Sweating (only horses)
-increase Na and H2O los -dehydration -drink H2O -inc. renal retention of H2O -hyponatremia |
|
|
3rd Space loss causing hyponatremia
-pathogenesis |
uroperitoneum
-Na poor urine enters the peritoneal cavity -plasma Na diffuses down the conc. gradient and into the peritoneal cavity -hyponatremia |
|
|
General methods of Na loss leading to hyponatremia
|
-renal loss
-intestinal loss -cutaneous loss -third-space loss |
|
|
Blood glucose conc. to cause a H2O shift from ICF to ECF
|
- > 400 mg/dl
|
|
|
Marked Hyperglycemia causing hyponatremia
-pathogenesis |
-H2O leaves cells
-dilutes Na in ECF -hyponatremia -glucosuria -diuresis -inc. renal Na loss -hyponatremia |
|
|
Mechanisms for Normonatremia disorders
|
-inc. Na & H2O retention (edema and/or transudates)
-net Na & H2O loss from normonatremic dehydration |
|
|
Reasons for inc. Na and H2O retention causing edema and/or transudates in normonatremia
|
-heart failure
-cirrhosis -nephrotic syndrome |
|
|
How do you differentiate normonatremic disorder and hyponatremic disorder with concern to inc. Na and H2O retention?
|
-hyponatremic is due to H2O retention > Na retention
|
|
|
Causes of normonatremic dehydration
|
-renal loss (renal disease, diuretics, obligate loss with anions)
-intestinal loss (diarrhea, intestinal sequestration) -cutaneous loss (sweating in horses) |
|
|
Most common type of dehydration
|
-normonatremic dehydration
|
|
|
Potassium concentration
-altered by |
-acid-base status
|
|
|
Potassium shift with inorganic acidosis
|
-accumulation of H+ in ECF
-H+ shift into cells for equilibration -K+ into blood to maintain electrical neutrality |
|
|
Potassium shift with alkylosis
|
-H+ in ECF reduced
-H+ leaves cells and enters plasma -K+ leaves plasma and enters cells to maintain electrical neutrality |
|
|
Effect of excess Potassium loss on blood H+ concentration
|
-decrease in K+
-shift of K+ from cells to ECF -H+ moves from blood to cells to maintain electrical neutrality -alkylemia occurs |
|
|
Inorganic acids
-definition -examples |
-acids that do not contain carbon
-H3PO4, H2SO4, HCl |
|
|
Reasons renal failure causes inorganic metabolic acidosis:
|
-decreased excretion of H+ --> inc. H+
-dec. HCO3 (consumed to buffer inc. H+) -dec. excretion of H2PO4 & HPO4 --. hyperphosphatemia |
|
|
Organic acids
-definition -examples |
-acids that contain carbon
-lactic acid -acetoacectic acid -beta-hydroxybutyruc acid |
|
|
Effects that organic metabolic acidosis can have on potassium
|
1) increased H+ and anion produced and enters blood
-both H+ and anion enter cells due to concentration gradient -electrical neutrality is maintained so K+ does not leave the cell 2) H+ and anion are both produced and enter the blood -H+ moves into the cell, and K+ leaves the cell and enters blood to maintain electrical neutrality -K+ is excreted renally with anion -no hyperkalemia occurs |
|
|
Hyperkalemia occurs with
|
-renal failure causing metabolic acidosis (oliguria)
|
|
|
Regulation of Plasma K+
|
-diet containing cells (K+)
-primary route of excretion = kidneys (inc. aldosterone --> inc. kidney loss of K) -insulin/epinephrine --> inc. K+ into cells |
|
|
Major mechanisms for hyperkalemia
|
-shift from cells
-inc. total body K+ (dec. renal loss) |
|
|
Renal insufficiency causing hyperkalemia
-pathogenesis |
-Oliguria
-diminished rate of tubular flow through the nephron -secreted K+ accumulates in the tubular fluid, diminishing gradient -K+ secretion into the tubular fluid decreases and build up occurs in the plasma -hyperkalemia |
|
|
Uroperitoneum causing hyperkalemia
-pathogenesis |
-urinary tract leakage
-K+ enters ECF -K+ can't be removed from the body -hyperkalemia |
|
|
Hypoadrenocorticism causing hyperkalemia
-pathogenesis |
-adrenocortical hypoplasia decreases aldosterone and cortisol production
-hypoaldosteronemia -decreased activity of Na-K-ATPase pumps in collecting tubules -decreased resorption of Na & decreased secretion of K -increased K+ in blood -hyperkalemia |
|
|
Pseudohyperkalemia
-causes |
-In vitro hemolysis (cattle, horses)
-Thrombocytosis (marked) |
|
|
When can in vitro hemolysis cause pseudohyperkalemia?
|
-when RBC [K+] > Plasma [K+]
|
|
|
In what animals can In vitro hemolysis cause pseudohyperkalemia?
|
-horses
-cattle |
|
|
How does thrombocytosis causes a pseudohyperkalemia?
|
-blood collected
-marked coagulation occurs -clotting causes K+ to enter serum -pseudohyperkalemia |
|
|
General 2 ways that hypokalemia will occur
|
-K+ shift into cells
-dec. in total body K+ |
|
|
Ways to decrease total body K+
|
Dec. Intake:
-anorexia Inc. Loss: -renal -intestinal -cutaneous |
|
|
Shift of K+ into cells causing hypokalemia
-pathogenesis |
metabolic alkalosis
-hypovolemia activates RAAS increasing renal secretion a K+ -bicarbonaturia causes renal excretion of cations including K+ -decreased dietary intake |
|
|
anorexia leading to hypokalemia
-pathogenesis |
-anorexia
-dec. K+ intake with concurent daily loss of K+ via kidneys & intestine -dec. total body K+ -hypokalemia |
|
|
Length of time for hypokalemia to develop in food restricted:
-dogs and cats -herbivores |
-dogs and cats: 2-3 days
-herbivores: longers |
|
|
Ways increased renal excretion can cause hypokalemia
|
-polyuria
-ketonuria (anions cause the obligate excretion of cations/K+) |
|
|
Ways increased intestinal loss can cause hypokalema
|
-diarrhea
-sequestration of intestinal fluid (equine) |
|
|
Inc. cutaneous sweat
-pathogenesis |
Cutaneous loss
-inc. K+ loss -hypokalemia -inc. H2O loss -dehydration -stimulate thirst -stimulate ADH -H2O dilutes K+ -hypokalemia |
|
|
In what animal is [K+] higher in sweat than in plasma
|
-horse
|
|
|
When does normokalemia typically occur
|
Acidotic states
-dec. tb K+ -acidosis --> K+ shift from ICF to ECF |
|
|
Sodium Potassium ratio
|
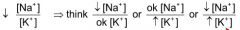
|
|
|
Classic cause for a decreased Na/K ratio
|
-hypoadrenocorticism (inc. Na loass w/ inc. K retention)
|
|
|
Reasons for a decreased Na/K ratio
|
-hypoadrenocorticism
-diarrhea/hemorrhage -renal failure -urinary tract obstruction -diabetes millitus with ketonuria -3rd space loss of Na -other |
|
|
Ways [Cl-] is controlled
|
-renal resorption and excretion
-alimentary tract function |
|
|
Renal effects on [Cl-] control
|
-Na+ and Cl- resorption
-NH4+ and Cl- secretion -HCO3- and Cl- exchange |
|
|
Alimentary Tract effects on [Cl-] control
|
-H+ and Cl- resorption and secretion
-Na+ and Cl- resorption -HCO3- and Cl- exchange |
|
|
General reasons why hyperchloremia may occur
|
-[Na+] goes up --> expect inc. [Cl-]
-[HCO3-] goes down --> expect inc. [Cl-] or increased [anion conc.] |
|
|
Hyperchloremia due to Hypernatremia
-reasons |
-Loss of H2O with Na content (diabetes insipidus, osmotic diuresis, osmotic diarrhea)
-pure H2O loss as water vapor |
|
|
Hyperchloremia due to Proximal Renal Tubular Acidosis
-pathogenesis |
-Proximal tubular defect
-HCO3- lost in urine -Acidosis from loss of HCO3- -Cl- retained to maintain electrical neutrality -Hyperchloremic metabolic acidosis |
|
|
Hyperchloremia due to Distal Renal Tubular Acidosis
-pathogenesis |
-Distal tubular defect
-impaired H+ excretion -HCO3- consumption for buffer -Reduced Cl- excretion for electrical neutrality -Hyperchloremic metabolic acidosis |
|
|
Hyperchloremia due to diarrhea
-pathogenesis |
-secretions contain Na+ and HCO3-
-fluid loss -HCO3- depeletion -Cl- fluid left behind -hyperchloremia |
|
|
In what animals is diarrhea and important cause of hyperchloremia?
|
-horses
|
|
|
General reasons for hypochloremia
|
-hyponatremia
-increased bicarbonate -increase in unmeasured anions |
|
|
Hypochloremia from hyponatremia can be due to
|
-edematous disorder
-dehydration disorder -uroperitoneum -marked hyperglycemia |
|
|
Hypochloremia due to an edematous disorder
-pathogenesis |
-retention of H2O
-dilution of Na+ & Cl- |
|
|
Routes that dehydration can occur
|
-renal loss
-intestinal loss -cutaneous loss (sweating in horses) |
|
|
Hypochloremia due to a dehydration disorder
-pathogenesis |
-excretion of Cl- with Na+ and H2O via kidneys, intestines, skin
-H20 moves into vasculature to compensate for hypovolemia -dilution of electrolytes -hypochloremia |
|
|
Hypochloremia due to uroperitoneum
-pathogenesis |
-Na+ and Cl- reabsorbed in the renal tubules
-low Cl- content in urine -urine into peritoneal cavity -Cl- diffuses from the blood and into the peritoneal cavity -hypochloremia |
|
|
Electrolyte balance by the stomach/abomasum
|
-H+ secretion
-Cl- secretion -HCO3- production |
|
|
Hypochloremic metabolic acidosis due to vomiting
-pathogenesis |
-Cl- secreted by the stomach mucosa
-vomiting does not allow Cl- to enter the intestine -dec. Cl- in the blood -increase in HCO3- |
|
|
How does vomiting cause paradoxical aciduria?
|
-Vomiting causing loss of Cl-
-inc. in HCO3- in plasma -animal becomes hypovolemic -RAS system acitvated -inc. aldosterone production -inc. excretion of K+ and H+ |
|
|
Paradoxical Aciduria
-definition |
-aciduria in the presence of alkalosis
-excretion of hydrogen even though the body is alkalotic |
|
|
hypochloremia due to a metabolic acidosis
-pathogenesis |
-ketoacidosis/lactic acidosis
-increased filtration of unresorbable anions from plasma -Na+ excreted to maintain electrical neutrality -Cl- follows -hypochloremia & inc. HCO3- |
|
|
What can give a falsely high chlorine concentration in a sample?
|
-bromide
|
|
|
[HCO3-] is proportional to
|
-[tbCO2]
|
|
|
Carbonic Anhydrase locations
|
-erythrocytes
-renal epithelial cells -gastric parietal cells |
|
|
Inc. [HCO3-]
|
-metabolic alkylosis
|
|
|
Metabolic alkylosis causes
|
-conservation by the kidney
-abomasal (displacement, torsion, etc.) -vomiting (pyloric obstruction, etc.) |
|
|
Ways H+causing acidosis is removed from the body
|
-buffered by HCO3- and blown off as water w/ CO2 by the lungs
-form ammonium --> released by kidneys -form HPO3- --> releaased by kidneys -H+ --> released by kidneys |
|
|
How is urine pH measured?
-why is this important |
-measured by the amount of free H+ in the urine
-need to remember that that is H+ that is not counted bound to other molecules |
|
|
dec. [HCO3-]
|
-metabolic acidosis
|
|
|
dec. [HCO3-] mechanisms
|
-dec. renal loss of H+
-inc. H+ production -inc. HCO3- loss |
|
|
Dec. loss in H+
-causes |
-dec. renal loss (renal failure, urinary obstruction, uroperitoneum)
|
|
|
Inc. H+ production
-causes |
-hypoxia
-inc. beta-oxidation of fatty acids |
|
|
Dec. [HCO3-] due to hypoxia
-pathogenesis |
-hypoxia
-anaerobic glycolysis -lactic acidosis -increased degredation of ATP -inc. H+ |
|
|
Inc. HCO3- loss
-causes |
-renal tubular acidosis
-diarrhea (horses) -ruminant choke (can't swallow saliva) |
|
|
metabolic acidosis due to Inc. HCO3- loss can also be called
|
-secretory acidosis
|
|
|
Anion gap
-purpose |
-detect an increase in unmeasured anions
|
|
|
Anion gap
-equation |
Anion gap = [mC+] - [mA-] = ([Na+] + [K+]) - ([Cl-] + [HCO3-])
|
|
|
Major cations
|
-sodium
-potassium |
|
|
Major anions
|
-Chloride
-Bicarbonate |
|
|
Unmeasured anions
|
-proteins
-organic anions -phosphates -sulfates |
|
|
Why is a normal anion gap near 16-20 mmol/L?
|
-protein
|
|
|
Effect of hypoalbuminemia and hypoproteinemia on anion gap
|
-dec. anion gap
|
|
|
Organic Anions
-examples |
-lactate-
-ketone bodies -ethylene glycol |
|
|
What must be true if there is no change in the [cations] and there is an increase in the [organic anions]?
|
-decrease in either [Cl-] or [Hcl-]
|
|
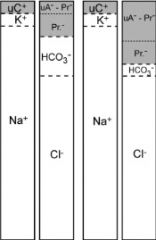
|
Normochloremic Metabolic Acidosis
|
|
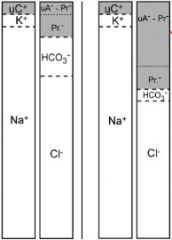
|
-hypochloremic metabolic acidosis
|
|
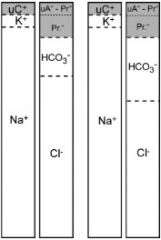
|
-hypochloremic metabolic alkalosis
(occurs with vomiting or GI sequestration of H+ and Cl-) |
|
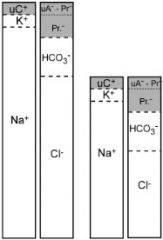
|
-hyponatremia and hypochloremia
|
|
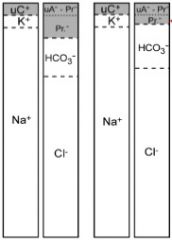
|
-hypoproteinemia
|
|
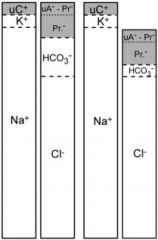
|
-pseudo-metabolic acidosis
-falsely increased AG |
|
|
Conditions causing an increased AG
|
Metabolic acidoses:
-lactic acidosis -ketoacidosis -renal failure -ethylene glycol (glycolate, oxalate) |
|
|
Lactate sink
|
-erythrocytes
|
|
|
major source of lactate in health
|
-muscles breaking down glucose
|
|
|
Cori cycle
|
-glucose to lactate via anaerobic glycolysis in peripheral tissue/skeletal muscle
-lactate to glucose via gluconeogenesis in the hepatocytes |
|
|
Formation of Lactate and Acidemia during hypoxia
-pathogenesis |
hypoxia
-anaerobic glycolysis -increased lactate production -production of lactate > liver use -increased [lactate] Hypoxia -anaerobic glycolysis -inadequate ATP production -ATP degredation -inc. H+ production -acidemia |
|
|
How should L-lactate in blood be measured?
|
-either using blood or plasma
-process it quickly so that erythrocytes do not add more L-lactate to the sample -decrease L-lactate production by collecting into a tube with NaF or by chilling -collect from free flowing blood to decrease lactate collection from stagnant blood |
|
|
Hyperlactemia
-causes |
-hypoxia disorder
-metabolic disorder -In vitro |
|
|
Hypoxia disorders that result in hyperlactemia
|
-stagnant hypoxia (shock, blood vessel occlusion)
-demand hypoxia (strenuous exercise, struggling in restraint) -hypoxemia (respiratory disorder) -hemoglobic hypoxia (anemia, methemoglobinemia) |
|
|
Lactic Acidosis
-definition |
-Lactate + H+ produced from the use of ATP
|
|
|
Metabolic disorders that result in hyperlactemia
|
-grain overload
|
|
|
Grain overload causing hyperlactemia
-pathogenesis |
-Excess starch intake
-increased formation of L-lactate by ruminal bacteria -increased lactate absorption by ruminal mucosa -increased plasma [L-lactate] |
|
|
In vitro hyperlactemia
-pathogenesis |
-stored blood
-erythrocytes continue glycolysis during storage -L-lactate production -inc. plasma [L-lactate] |
|
|
How a beta-hydroxybutyrate and acetoacetate produced?
|
-ketogenesis in the liver
|
|
|
Beta-hydroxybutyrate
-samples for assays |
-serum
|
|
|
Acetoacetate
-samples for assays |
-urine
-blood -serum -milk |
|
|
Clinical disorder involving increased ketone bodies
|
-ketosis
-ketonemia (blood) -ketonuria (urine) |
|
|
Conditions causing ketonemia
-all mammals |
-starvation
-prolonged anorexia -diabetes mellitus |
|
|
Conditions causing ketonemia
-cattle |
-high energy demands (lactation, lipidosis, diabetes mellitus)
|
|
|
Conditions causing ketonemia
-dogs |
-high energy demands (lactation, Diabetes mellitus, endurance)
|
|
|
Conditions causing ketonemia
-horses |
-Diabetes mellitus
-endurance racing |
|
|
Starling's Law
|
Pressure gradient = (Pcap - Pif) - (Oncotic cap - Oncotic if)
|
|
|
Why does water and electrolytes leave arterial capillaries?
|
-hydraulic pressure > oncotic pressure
|
|
|
Why does water and electrolytes enter venous capillaries?
|
-oncotic pressure > hydraulic pressure
|
|
|
Why is the osmolarity of the peripheral capillary bed not very different?
|
-difference is only really due to proteins
|
|
|
What are the biggest contributors to osmolarity?
|
-electrolytes
|
|
|
Increases in what can cause an increase osmolality (hyperosmolality)?
|
-inc. [Na] & inc. [Cl]
-inc. [urea] and/or inc. [glucose] -foreign substance |
|
|
Instrument used to measure osmolality
|
-freezing point osmometer
|
|
|
How does a freezing point osmometer work?
|
-the more solute that is present in the sample, the lower the freezing point will be
|
|
|
Hypoosmolality can be due to:
|
-dec. Na
-dec. Cl |
|
|
How can osmolality be calculated?
|
Osmc = 2[Na] + ([UN]/3) + ([glucose]/20)
|
|
|
In calculating osmolality, what does 2[Na] represent?
|
contribution of all electrolytes toward osmolality
|
|
|
Assays that measure Na+
|
-direct potentiometry
-indirect potentiometry |
|
|
Direct potentiometry
|
-no dilution of sample used for analysis
|
|
|
Indirect potentiometry
|
-dilution of sample during analysis
|
|
|
Pseudohyponatremia can be caused by:
|
-lipemia
-inc. [TP] |
|
|
How can lipemia or inc. [TP] cause a pseudohyponatremia?
|
-occurs in indirect potentiometry
-lipemia or inc. [TP] causes there to be less water in the sample -H2O portion of the sample becomes more diluted for analysis -same dilution factor is used from a sample w/o lipemia -hyponatremia |

