![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
208 Cards in this Set
- Front
- Back
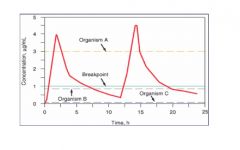
explain this graph
|
the red liine is the drug concentration in vivo. the break point is determined for each drug and is compared to minimum inhibitor concentration for each organism. if the MIC is below the break point the organism is susceptible if above the organism is resistant.
|
|
|
what are the eskape pathogens
|
these are the most common antibiotic resistant microrganism
Enterrococcus facium staph aureous klebsiella pnemonia acinetobacter baumani pseudomonias aeruginosa enterobacter species |
|
|
what are the synergistic drug combinations for antibacterials
|
CWS + aminoglycosides
sulfonamides + folic acid reductase inhibitors quinupristin + dalfopristin |
|
|
what is stage 2 in the cell wall synthesis of bacteria
|
insertion of petidoglycan into the cell wall via the enzyme transglycosidase
|
|
|
what is the stage 3 in cell wall synthesis of bacteria?
|
transpeptidase facilitates cross linking of the peptidoglycans to strengthen the cell wall. by binding a 5 glycine side chain of one to a D alanin of another.
|
|
|
how do beta lactams inhibit cell was synthesis
|
they irreversibly bind transpeptidase.
|
|
|
what is required for beta lactams to effectively kill bacteria
|
bacterial growth. if no new cell wall is being made then beta lactam will not be able to effect the cell
some killing may still be taking place because they also stimulate some level of autolysis but this is not understood. |
|
|
what type of killing does beta lactam type drugs do.
|
time dependent
|
|
|
what are some examples of the beta lactams
|
penicillians
cephalosporins cehpalomycins carbapenems monobactams |
|
|
what are the drugs that kill during the second stage of the cell wall synthesis?
|
vancomycin
televancin |
|
|
what are the drugs that kill during third stage of the cell wall synsthesis
|
beta lactams
vancomycin televancin |
|
|
what is the primary cause of beta lactam resistance?
what are some secondary ones? |
1.production of beta lactamase.
2.altered penicillian binding protiens ie transpepidase.(MRSA) |
|
|
what is the active part of the beta lactams?
|
the beta lactam ring
|
|
|
what are some physiolgic barriers for efficacy of penicillan
|
not acid stable so much of it is degraded in the stomach.
very quickly excreted by kidneys through secretion t1/2 of only 30 mins this is often countered by adding probencid to block excretion |
|
|
what are the antistaphlococcal penicillians
|
oxacillian
nafcillian diclooxacillan methicillian all these have large sidegroups that prevent them from being degraded by beta lactamases produced in all staphlococci. |
|
|
what is the difference between nosocomial MRSA and community aquired MRSA
|
Nosocomial MRSA has plasmids that make it resistant to many types of antibacterials wherease community acquired only has the altered penicillan binding protiens so it is primarily resistant to only the beta lactams.
|
|
|
What can normal penicillian kill?
|
gram positives
|
|
|
What are the extended spectrum penicillans
|
these are penicillans that have been designed to be able to kill some gram negatives as well as gram positives.
ampicillian and amoxacillan |
|
|
what are the anti pseudmonia penicillans
|
penicillans that have enchanced killing abiilty against pseudomonas
ticarcillian piperacillian |
|
|
what are the beta lactamase inhibitors
|
these are drugs added to the normal beta lactamases that alllow them to be effective even against bacterial that produces beta lactamase
clavulinic acid sulbactam tazobactam |
|
|
what are the differences and similarities between cephalosporins and beta lactams
|
same MOA
same resistances except cephalosporins are not effected by beta lactamase they have an increased spectrum that allows them to be used against gram negatives. there are 5 generations of cephalosporins the later the generation the more effective vs gram neg generation 3 or greater can penetrate the CNS |
|
|
What are some examples of 1st generation cephalosporins?
|
cefazolin which is used against staph and strep.
only removed from body by filtration not secrection cefalexin - taken orally |
|
|
what are some examples of the 2nd generation cephalosporins?
what differentiates these from the 1st generations |
cefaclor
ceforoxime cefotetan these are more active against gram negatives more stable against beta lacatmases |
|
|
what are the 3rd gen cephalosporins?
|
ceftazidime
ceftriaxone cefotaxime |
|
|
ceftazidime is the drug of choice against?
|
psuedomonas
|
|
|
ceftriaxone is the drug of choice against?
|
N. gonorrhea
|
|
|
What is the only 4th gen cephalosporin?
|
cefepime
|
|
|
cefepime is the drug of choice for what?
|
nothing it is held back to treat resistant bugs.
|
|
|
what is the only 5th gen cephalosporin?
|
ceftaroline
|
|
|
ceftaroline is used for?
|
MRSA, and highly penicillian resistant s. pneumoniae.
the only beta lactam effective against MRSA |
|
|
What are the carbapenams?
|
the broadest spectrum beta lactam group.
effective against bugs that are resistant to penicillan and cephalosporins. there are some klebsiella pneumoniae that are resistant Imipenem meropenem ertapenem |
|
|
What drug is mixed into all preparations imipenam and why?
|
cilostatin is mixed in, in order to prevent degradation of imipenam in the intestines prior to absorbtion.
|
|
|
What are the monobactams?
|
only effective against aerobic gram neg.
resistant to most of the beta lactamases so there are not any bugs that are resistant to it no cross sensitivity with the other beta lactams. so if they are allergic to penicillan they will not be allergic to this most other beta lactams have cross sensistiviy. aztreonam |
|
|
What are some adverse reactions to the penicillans
|
hypersensitivity mediated by IgE.
cross sensitivity if allergic to one ur allergic to all. {nephritis hemolytic anemia thrombocytopenia leukopenia }all caused by immune response IgG and IgM impaired platlet aggregation- only in ticaracillan electrolyte disterbances siezures ampicillan rash- almost 100% in CMV patients this is not an allergic reaction so drug can continue to be given. not readily able to differentiate this with the allergic rash though. |
|
|
What is major and minor haptan?
|
they are products of allergic reactions in the body to penicillan. you can test for the major haptan but not the minor one.
|
|
|
What are some adverse reactions to the cephalosporin?
|
hyerpsensitivity rxn
5-10% chance of cross sensititivey with penicillans rare renal damage cefoperazone and cephotetan can cause hyoprothrombopenia and bleeding as well as cause a anatbuse syndrome-vomit with alcohol |
|
|
What are some adverse reactions to the carbopanams
|
hypersensititivy
cross with penicillan convulsions and siezures |
|
|
What is vancomycins MOA
|
glycopeptide inhibitor of cell wall synthesis
inhibits transglycosidase binds to D-alanine-d-alanine |
|
|
What is vancomycins used for
|
antibacterial Gram positives
MRSA resistant staph highly resistant s. pneumonaie. |
|
|
What are the adverser rxn of vancomycins?
|
redman(redneck) syndrome- infusion reaction causes granulation which causes redness
{ototoxicity nephrotoxicity} not common anymore except in people arready comprimised |
|
|
Televancin is different than vancomaycnin how?
|
has a lipid tail that inserts into the membrane of the bug as well as binding the d-alanine d-alinine
displays concentration dependent killing as opposed to vancomycin which only does time dependent killing |
|
|
Bacitracin is used how?
|
topically
works against cell wall formation stage 1 |
|
|
cycloserine is used how?
|
against drug resistant TB
works against cell wall formation stage 1 |
|
|
fosfomycin is used how?
|
UTIs
works against cell wall formation stage 1 |
|
|
What is the difference between bacterial and human protein synthesis?
|
bacterial use 70s ribosomes and human use 90s ribosomes
exception is that mitochondria use 70s ribosomes. therefore drugs that target bactierial protien synthesis can effect human mitochondria. |
|
|
What subunits make up the 70s bacterial ribosome?
|
50s and a 30s
|
|
|
What are the 50s inhibitors?
|
macrolides
ketolides lincosamides nitroaromatics streptogramins oxizolidinones all these work to inhibit peptide bond formation |
|
|
what are the 30s inhibititors?
|
aminoglycosides
tetracylclines tiglycyclines these interfere with mRNA decoding |
|
|
What is the 50s subunit made up of?
|
a 23s unit and a 5s unit as well as 32 proteins.
|
|
|
the 23s unit of the 50s subunits has how many domains?
what domain is closest to domain V |
6
domain II |
|
|
What is the mech of action of the macrolides?
|
they bind to adenine in the domain V on the 23s portion of the 50s subunit
|
|
|
What is the cause of resistance in macrolides?
|
mutation of the adenine in the Domain V.
methylation of the adenine efflux pump which is coded by the mef gene major form of resistance in the US |
|
|
What are some examples of the macrolides?
|
erythromycin
clarithromycin azithromycin |
|
|
What are some bugs that erythromycin is effective against?
|
strep
staph clymidia pnemoniae campylobacter legonella often cross resistance with the penicillians |
|
|
Erythromycin and clarithromycin can have drug interactions with what?
|
carbamazepine
theophyline bc Erythromycin and clarithromycin inhibit p450. |
|
|
what are some similarities and differences between erythromycin and clarithromycin?
|
clarithromycin is more GI stable and has higher tissue concentration but it is also degraded by p450.
|
|
|
what are the advantages of azithromycin over the other macrolides?
|
extremely high tissue concentration
no GI issues not metabolized by p450 effects more bugs than the other two. |
|
|
What are some adverse effects of macrolides/
|
fairly safe drugs
cholestatic hepatitis(erythromycin) Epigastric distress(erythromycin) acute psychosis(clarithromycin) prolongation of the QT interval. |
|
|
What is the major difference in MOA btw the macrolides and the ketolides?
|
the ketolides bind to domain V but they also bind to domain II.
this means that the resistance mutation that effect the macrolides dont effect the ketolides, the ketolides are also unaffect by the efflux pump. |
|
|
What is an example of the ketolides/
|
telethromycin
|
|
|
what are some adverse effects of the ketolides?
|
severe liver failure and damage.
|
|
|
What are the ketolides used for?
|
community aquired pneumoniae.
|
|
|
what is an example of the lincosamides?
|
clindamycin
|
|
|
What are the similarities and differences btw the lincosamides and the macrolides?
|
they both have a similar MOA
methylation of the alanine will cause resistance to the lincosamides, but unlike the macrolides lincosamides do not induce methylation. |
|
|
What is the D-test
|
when put in an agar plate that has a bug that is resistant to erythromycin and inducible if the erythromycin is adjacent to the clindamycin it will induce resistance to the clindamycin which creates a D shape in the clindamycin area. when this happens the drug is considered resistant to clindamycin.
|
|
|
What is clindamycin used in?
|
staph
strep main use is with anerobic organisms(b.fragilis) very high affinity for bone tissue so used to treat osteomyelitis. |
|
|
What are streptogramins used for?
|
gram positives
MRSA methecillin susceptible staph multi drug resistant S. pnemoniae VRE facium this drug is bacteriacidal as opposed to bacteriastatic which is what most of the other drugs are. |
|
|
What is the main streptogramin
|
synercid which is quinopristin and dalfopristin mixed drug.
|
|
|
what are the adverse reaction of the streptogramins?
|
nausa vomiting diarhhea
myalgia and arthralgias must be administered via central line metabolized by and inhibits CYP3A4. |
|
|
What is an example of the oxazolanides
|
linezolid
|
|
|
what is linezolid used for?
|
gram positives
methicillan resistant and susceptible staph pen. resist. and susept. pneumonaie all enterrococci including VRE. |
|
|
What are some advantages of linezolid over other drugs
|
100% oral availablity
no effect on p450 |
|
|
what are some adverse effects of linezolids?
|
headache
nonselective MAOI myelosuppression peripheral neuropathy. |
|
|
What is the important info on chloramphinocol?
|
it is a good antibiotic with good oral availablity, but it is only used as a last resort if all other antibiotics have failed.
there are three major adverse reactions that can occur. myelosuppression aplastic anemia(very dangerous often fatal even months after stopping it) grey baby syndrome( babies cant metabolize this) |
|
|
What is the MOA of the tetracylines
|
pumped into the cell where they bind the 30s subunit and prevent mRNA decoding
|
|
|
What causes restance to the tetracylcines?
|
the presence of a pump to pump tetracylcine out of the cell.
|
|
|
What are tetracylcines used against?
|
broad spectrum antibiotic
ricketssia chlamidia mycoplasm H pylori S pneumonaie Staph aureus |
|
|
What are the three tetracyclines?
|
tetracycline
minocycline doxycyline |
|
|
what is the major difference among the tetracyclines?
|
minocycline and doxycycline both have a half life of 16-18 hours where as tetracycline has a half life of 6-8 hours so minocycline can be taken less often.
|
|
|
What are some adverse reactions among the tetracyclines
|
hypersensititivey rxn
-cross sensitivity GI irritation decreased absorption of -Ca,Al,Mg,Fe phototoxicity vestibular toxicity effects on bones and teeth -malformation -yellowing hyperpigmantation |
|
|
Tigecycline information?
|
derivative of minocycline
not many bugs are resistant used for skin, abdominal, and community aquired infections adverse effects are nausea and vomiting no drug interactions |
|
|
What type of antibiotic are the aminoglycosides?
|
bacteriacidal
|
|
|
What is the MOA of aminoglycosides?
|
entry into cell via oxygen dependent transport slow or energy dependent transport which is fast.
cause misread of mRNA |
|
|
What is the major resistance pathway for aminoglycosides?
|
metabolism by the bacteria via acetylation
|
|
|
how are the aminoglycosides eliminated?
|
via the kidney
they reside predominantly in the extrecellular fluids. |
|
|
What are the main three aminoglycosides?
|
gentamicin
tobramicin amikacin |
|
|
what are the two dosing mechanisms for aminoglycosides?
|
loading dose plus maintanence 3 dose per day
or one dose per day -prefered bc it increases concentration dependent killing and takes a long time for bugs to start growing again. cant be used in cystic fibrosis increased volume distribution decreased renal function bacterial endocarditis |
|
|
what are the adverse effects of aminoglycosides?
|
ototoxicity
nephrotoxicity neuromuscular blockade |
|
|
Alteration of penicillin binding proteins causes resistance to what drug?
|
Classes: Penicillins, Cephalosporins, Carbapenems, Monobactams
|
|
|
Alteration of penicillin binding proteins occurs in what bacteria?
|
MRSA, S. pneumonia
|
|
|
Penicillinase production causes resistance to what drug?
|
Penicillins (except Methicillin, Oxacillin, Dicloxacillin, Nafcillin), Cephalosporins (Resistance increases with class number), Monobactams
|
|
|
Penicillinase production occurs in what bacteria/
|
staph aureus. S. epidermis
|
|
|
alterations in Porin size affects what drugs?
|
: Penicillins (Ampicillin, Amoxicillin, Carbenicillin, Ticarcillin, Piperacillin); Cephalosporins (Cefotaxime, Ceftazidime, Ceftriaxone, Cefepime), Monobactams, Flouroquinolones
|
|
|
Acquisition of genetic material from environment creates drug resistance in what bacteria?
|
s. pneumoniae and N. meningitides
|
|
|
Sub of termrinal residue in peptidoglycan cauases resistance to? in what?
|
Vancomycin, telavancin
enterococci |
|
|
Bacterial efflux pump causes resistance to? in what?
|
Tetracyclines, macrolides, (Erytrhomycin, Clarithromycin, Azythromycin
pseudomonas aeruginosa |
|
|
mutation of ribosomal binding site causes resistance to what?
|
macrolides, (Erytrhomycin, Clarithromycin, Azythromycin
|
|
|
Acetylation of drug causes resistance to what?
|
Aminoglycosides ( Gentamicin, Tobramicin, Amikacin), Chloramphenicol
|
|
|
what is the MOA of the fluoroquinolones?
|
inhibit DNA gyrase in bacterail cells
DNA gyraes is equivialent to toposomerase II and it also targets topoisomerase IV |
|
|
how does resistance to fluoroquinolones occur
|
mutatino in the A subunit
mutatino of the B subnint change in porins all for DNA gyrase. |
|
|
What type of killing does fluorquinolones do?
|
Concentration dependenct killing
only other drug covered so far that does that is the aminoglycosides |
|
|
What is the range of killing for the fluoroquinolones?
|
gram neg
and gram pos |
|
|
what are some examples of the fluoroquinolones?
|
ciprofloxacin
levofloxacin moxifloxacin |
|
|
what limits fluoroquinolones absorption
|
metal ions
|
|
|
how is fluorquinolones eliminated
|
renal
|
|
|
what are some adverse effects of fluoroquinolones
|
generally well tolerated
CNS: headache, dizziness, drowsiness, confusino, insomnia, fatigue, malaise, depression, somnolence, siezures, vertigo, lighheadedness, restlessness, tremor alergic rxn tendon rupture(black box) no exercise for week after treatment potential cartilage damge in children hypoglycemia in glyburide |
|
|
What is an example of Rifamycin
|
rifabutin
|
|
|
what is the MOA of rifabutin
|
inhibit bacterial DNA dependent RNA polymerase
|
|
|
is Rifabutin bacterial cidal or static
|
cidal
|
|
|
what is the major drug interaction from Rifabutin
|
anything broken down by CYP3A4
it is a potent inducer of CYP3a4 |
|
|
What are the antimetabolites?
|
sulfonamides
trimethoprim |
|
|
what is the moa of antimetabolites
|
interfere with essential metobolic pathway ofthen folic acid pathway used in the sulfonamides
|
|
|
Why are sulfonamides selectively toxic toward bacterial if they target folic acid
|
there is a difference in teh souce of folic acid for humans and bacteria
humans-dietary requriement bacteria-biosynthesis |
|
|
How does bacteria form folic acid?
|
pteridine + PABA transformed into folic acid by two enzymes
dihydropteroate synthase dihydrofolate reductase |
|
|
What is the MOA of sulfonamides
|
inbhibits dihydropteroate synthase by imitating PABA
|
|
|
What are some of the mechanism of resistance for sulfonamides
|
altered dihydropteroate synthatase
over prodcution of PABA utilization of the exogenous folic acid |
|
|
what are sulfonamides,Sulfisoxazole, sulfacytine) used for
|
UTIs
|
|
|
Trimethoprim is a what?
|
antimetabolite inhibits folic acid reductase
didyrofolate reductase inhibitor |
|
|
what is Trimethoprim used for
|
combonation with sulfonimades for UTS
combonation is synergistic and bacteriacidal upper respiratory tract MRSA |
|
|
what are teh adverser effects of sulfonamides and trimethoprinm
|
allergic rxn
mild to MAJOR rash called steven johnson syndrome cross sensitization allergic to one allergic to all drug fever blood dyscrasias drug induced hepatitis kernicterus trimethoprim can interfere with folate metabolism in malnourished indiviuals(alcholics) |
|
|
what is an example fo the unrinary tract antibiotics
|
nitrofurantoion
methenamine fosfomycin |
|
|
what are some adverse effects of nitrofurantion
|
GI nausea and vomiting
acute allergic reaction chronic cough, dyspnea pulmonary fibrosis |
|
|
what is an example fo the lipopeptides antibacterials
|
daptomycin
|
|
|
what is the moa of daptoycin
|
inserts into the cytoplasmic memebrand and creates a hole that lets out the K
|
|
|
what type of killing does daptomycind have
|
concetration dependent
|
|
|
what is daptomycind used for
|
MSRA
VRE |
|
|
What is metronidazole used for
|
cdif
hpylori bfragilis penetrates the BBB to treat brain infection |
|
|
adverse effects fo metronidazole
|
metallic taste
alchol throw up headache |
|
|
what is the use of spectinomycin
|
N. gonorrhoeae
alternative to cephalosporin |
|
|
*what antibacterials are only effective against gm+*
|
vancomycin
telavancin bactriacin quinupristin/dalfopristin linezolid daptomycin |
|
|
** what antibacterials only work against gm-**
|
aztreonam
polymyxin B polymyxin E(colistin) |
|
|
what is the cornerstone of treatment of tuberculossis
|
use of multidrug regimens
bc it mutates so fast if u use only one it will become resistant before it is wiped out |
|
|
what are the major drugs used to treat TB
|
isoniazid
rifampin ethambutol pyrzinoamide streptomycin |
|
|
what is the most important TB drug
|
Isoniazid
MOA activation of catalase/peroxidase inhibition of mycolic acid synthesis(component of cell wall) |
|
|
what is the resistance of isoniazid
|
loss of catalase/peroxidase gene
|
|
|
What is important about the metabolism of isoniazid
|
N-acetylation
some people are slow acetylators which means they will have higher serum levels of the drugs. which leads to peripheral neuropathy |
|
|
what are the major adverse effects
|
peripheral neuphathy-treated with vit B6
isoniazid induced hepatitis |
|
|
what is the second most important TB drug and MOA
|
rifampin
moa inhibits bacterial DNA dependent RNA polymerase |
|
|
what are some adverse effects of rifampin
|
orange urine and secretion
potent inducers of CYP3a4 |
|
|
what are some adverse effects of ethambutal TB drug
|
ocular toxicity
hyperuricemia |
|
|
What are some adverse effects of strpetomycind
|
ototoxic(vestibular-balance)
nephotoxic |
|
|
What are some drugs used to treat leprosy
|
dapsone
rifampin' clofazimine |
|
|
what is the most important target in antifungal drugs
|
ergosterol
|
|
|
what is the human equivalent of ergosterol?
|
cholesterol
|
|
|
What is the moa of the polyene antifungals
|
binds to ergosterol and changes membrane permiablity
|
|
|
what are some examples of the polyenes
|
nystatin
amphotericin B |
|
|
what is the use of nystatin
|
local administration for thrush
too toxic to take systemicly |
|
|
what is the use of amphtericin B
|
gold standard antifungal broad specturm funcicidal and fungistatic
also toxic but not as bad as nystatin |
|
|
what are the side effect of amphotericin B
|
nausea
vomintin fever chill arthralgia myalgia heaaches thromobphebitsi hypotension nephrotoxicity |
|
|
what is the moa of the azole antifungals
|
inhibits lanosterol 14alpha demethylease which is an enzyme in ergosterol synthesis.
|
|
|
what are some mechanism of reistance for azoles
|
altered target protein over espresion of the target efflux pumps
|
|
|
what are some examples of azole antifungals
|
ketoconazole
fluconazole itraconazole voriconazole |
|
|
what are some adverse effects of ketoconazole
|
large doses inhibit human sex steroid
P450s inhibits CYP3a4s high stomach acidity for absorption drink coke increases absorption h2 blockers decrease absorption |
|
|
what are some adverse efffects of itraconazole
|
inhibits CYP3a4
but not P450s |
|
|
what is the advantage of fluconazole over itraconazole
|
least likely to inhibit drug metaoblism high bioaviality
high level can be teratogenic |
|
|
what is the use of voriconazole
|
invasive resistant aspergillosis
|
|
|
what is the moa of allylamines antifungals
|
inhibitors of squalene epoxidase and P450s which is an early enzyme in the ergosterol pathway
|
|
|
what is an example of allylamines
|
terbinafine
|
|
|
what is synergistic with the azoles
|
terbinafine
|
|
|
What is the moa of flucytosine antifungal
|
inhibits cytosine deaminase which inhibits protien synthsis and DNA sysnthsis
|
|
|
how is flucytosine used
|
synergistically with amphotericin B
bc resistance develops rapidly when taken alone |
|
|
what is the adverse rxn of flucytosiine
|
potentially fatal bone marrow depression
hepatic dysfunction |
|
|
what is the moa of echinocandinds
|
inhibits fungal cell wall synthesis
|
|
|
what are the examples of echinocandins
|
casofungin
anidulafungin micfungin |
|
|
what are drugs that inhibit influenze replication
|
amantadine
ramantadine neuroaminidase inhibitors |
|
|
what is the moa of amantadine and ramantadine
|
block acidification of Ion Channel which prevents release of RNA from virus
|
|
|
what is the use of amantadine and rantaiden
|
influenza A
|
|
|
what is the advsere effect of amantadiene
|
cns stimulation in elderly
|
|
|
what is the moa of neuraminidsase inhibitors
|
prevents the neuramindase on the virus from removing the cyalic acid residues from teh cell surface which leads to a clumping of the virus particals and this make them noninfective
|
|
|
what are some examples of neuraminidase inhibitors
|
zanamivir
oseltamivir |
|
|
what are neuraminidase inhibitiors effective against
|
influenza A and B
|
|
|
what is the broad sprectrum antiviral
|
RIbavirin
|
|
|
what is the prototype of most nucleoside analog antivirals
|
acyclovir
|
|
|
what is the moa of acyclovir
|
acyclovir is phosphoralated only in virus infected cells once phospohralted it is a competitive inhibitor of viral DNA polymerase
|
|
|
what is a mech of resistance against acyclovir
|
viruses that do not phosphorylate the acycolvir
|
|
|
acyclovir is only effective against what
|
actively reproducing viral infections
|
|
|
what is the use of trifluridine
|
local application in eye fro herpies simplex infection of the eye
|
|
|
what is an example of the non nucleoside antivirals
|
foscarnet
|
|
|
what is the moa of foscarnet
|
binds to phosphate binding sidte of polymerases
|
|
|
what is the toxicity of foscarnet
|
100% headaches
100% fatigue 80% nausea 33% renal impairment |
|
|
what are the classes of drugs that are used to treat HIV
|
nucleoside reverse trnascriptase inhibitors
non nucleoside reverse transcriptase inhibitors HIV protease inhibitors intgrase inhibitors fusino inhibitors entry inhibitors |
|
|
what is the problem with monotherapy with AZT
|
HIV virus rapidly develops rsistance
|
|
|
what are some example of the nucleoside RTI(reverse Transcriptase inhibitor)
|
zidovudine
stavudine lamivudine didanosine emtricitabine tenofovir abacavir |
|
|
what is the moa of nucloside RTIs
|
turned to triphosphate by viral proteins
competative inhibits HIV RT |
|
|
what is the nRTI class toxicity
|
inhibition of mitochondrial function
lactici acidosis anemai granulocytopenia periopheral neuophathy myophathy lipatrophy |
|
|
What are the class specific toxicty of the nnRTIs
|
rash
hepatotoxicty |
|
|
what are examples of the nnRTIs
|
nevirapine
efavirenz |
|
|
what is the moa of nnRTIs
|
do no require activation
only active against HIV1 bind to RT and make it inactive metabolized by CYP3a4 |
|
|
what is the contraindication for efavirenz
|
cns effects leads to psychosis
and its teratogenic so not used in pregnant women |
|
|
what is the moa of the portease inhibtors
|
prevents maturation of the virus by inhibtioin of the protease
|
|
|
what are some adverse reaction of protease inhibitors
|
GI
CYP3a4-inducers inhibitor and metabolized this leads to a lot of drug interactions |
|
|
what are some examples of protease inhibitors
|
atazanavir
ritonavir rosamprenavir darunavir lopinavir sawuinavir |
|
|
what is an exampleo f integrase inhibitors of HIV
|
raltegravir
|
|
|
what is the moa of raltegravir
|
blocks insertion of HIV DNA into host DNA
|
|
|
what is an example of fusion inhibitors for HIV
|
enfurvirtide
|
|
|
what is the moa of enfurvittide
|
targets gp41
which prevents binding to cd4 so HIV not uptaken |
|
|
what is an example of entry inhibtors
|
maraciroc
|
|
|
what is the moa of maraviroc
|
binds to CCR5 which is a human protein and it prevent uptake of the HIV
|
|
|
what are some long term effects of anti HIV therapy
|
lipodystrophy syndrome
- fat redisribution elevated cholesterol elevated triglycereides hyperglycemia insulin resistance |
|
|
what are the stages of maleria
|
protozoa leave mesquito go to liver grow there and are released there as merozoites which go and get into the RBCs they grow in there into trophozioes and then schizont and then rupture the RBC and release more merozoites
|
|
|
what are the 4 types of malaria
|
plamodium falciparum
plamodium milariae plasmodium vivax plasmodium ovale |
|
|
what are the differences in the 4 types of malaria
|
p. vivax and p ovale can incubate in the liver for long periods of time for later reactivation of the disease
P. falciparum causes the most severe for the disease and the most common drug reistant species |
|
|
what is the most common chemoprevenion for malaria
|
chloroquine for all except p falcium that is resistance
malarone is used for the resistant one mefloquine doxycycline primaquine is used to kill the liver hyponozoites that may still remain |
|
|
what is the treatment for malaria
|
chloroquine then primaquine
quinine plus doxycycline or clindamycin artesunate |
|
|
what are the adverse effects fo chloroquine
|
contraindicated in pt with psroriasis
and porphyrria |
|
|
what is the adverse effects of mefloquine
|
neuropsych problems
|
|
|
what is the moa of primaquine
|
only drug effective against liver hypnozoites
|
|
|
what is the major problem with primaquine
|
most potent inducers of hemolytic anemia in G 6 PDH deficient people
|

