![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Chemical bond |

|
|
|
|
Octet Rule |

|
|
|
|
Formal Charge F.C |

|
|
|
|
Electron Gain Enthalpy |

|
|
|
|
Lattice Enthalpy |

|
|
|
|
Bond length |

|
|
|
|
Bond angle |

|
|
|
|
Bond enthalpy |

Energy required to break 1 mole of bond |
|
|
|
Bond order |

|
|
|
|
Bond order and bond dissociation enthalpy |

Inverse relation |
|
|
|
Resonance structure |

|
|
|
|
Non polar covalent bond |

|
|
|
|
Dipole moment |

Charge into distance of seperation |
|
|
|
VSEPR Theory |

|
Valence Shell Electron Pair Repulsion Theory |
|
|
Repulsive interaction decreasing order |

|
|
|
|
Geometrical Name |
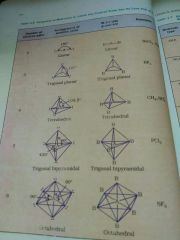
2 Linear 3 Trigonal Planar 4 tetrahedral 5 Trigonal bipyramidal 6 octahedral |
|
|
|
Geometrical name if Lone pairs Are present |

2,1,Bent 3,1,Trigonal pyramidal 2,2,bent 4,1,see saw 3,2,t-shape 5,1,square pyramid 4,2,square planar |
|
|
|
Relation |

|
|

