![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
|
Atoms
|

The smallest particle of an element.
|
|
|
Atomic Mass
|

The mass of an atom in atomic mass units; the average mass of the atoms of an element.
|
|
|
Atomic mass unit
|
One-twelfth the mass of atom.
|
|
|
Atomic number
|

The number of protons in the nucleus of an atom.
|
|
|
Atomic symbol
|

the number of positive charges or protons in the nucleus of an atom of a given element, and Therefore also the number of electrons normally surrounding the nucleus.
|
|
|
Nucleus
|
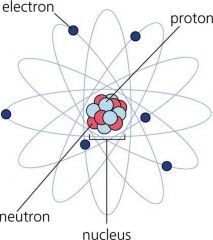
A central part about which other parts are grouped or gathered.
|
|
|
Period
|
A horizontal row of the periodic table.
|
|
|
Proton
|
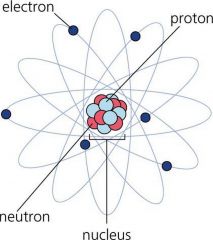
Positive nucleon.
|
|
|
Subatomic particle
|
A particle smaller than an atom.
|
|
|
Chemical Symbol
|
A notation using one to three letters to represent an element.
|
|
|
Electron
|
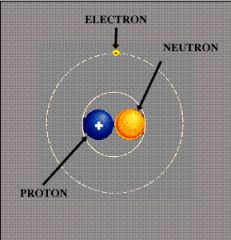
An elementary particle with unit negative charge.
|
|
|
Group
|
The elements of a vertical column in the prriodic table.
|
|
|
Isotope
|
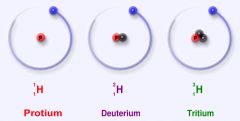
One of two or more atoms having the same number of protons but different numbers of neutrons.
|
|
|
Mass number
|

The total number of protons and neutrons in nucleus.
|
|
|
Metal
|
An element that tends to lose electrons in chemical reactions.
|
|
|
Metalloid
|
An element that has properties characteristic of a metal and a nonmetal.
|
|
|
Neutron
|

A neutral subatomic particle; a hadron
|
|
|
Nonmetal
|
An element that tends to gain elecetrons in chemical reactions.
|

