![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
Matter can be categorized as either... |
substances or mixtures. |
|
|
substance |
a material that has constant (unchanging) composition and distinct properties |
|
|
mixture |
a combination of two or more substances, each of which keeps its individual identities |
|
|
homogeneous mixture |
a mixture that has no visible boundaries between the substances that make it up, as a result, a homogeneous mixture has a uniform (changeless) composition throughout and is often called a solution |
|
|
heterogeneous mixture |
a mixture that has visible boundaries between the substances that make it up, as a result, the composition of a heterogeneous mixture varies
example: oil and vinegar dressing, granite, beefsteak are heterogeneous mixtures |
|
|
element |
a substance that cannot be broken down into simpler substances by chemical means; it is made of only one kind of atom
example: hydrogen, oxygen, iron, uranium |
|
|
compound |
a substance made up of two or more elements that are chemically joined in a fixed (uniform) composition
example: carbon dioxide, ammonia, hydrogen peroxide, sodium chloride (table salt), water |
|
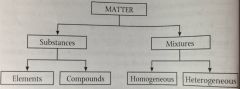
|
flowchart of matter |
|
|
How many chemical elements have been discovered so far? |
There are 115 chemical elements so far: 91 occur in nature and 24 have been made in laboratories |
|
|
How do you write the unique symbol of a chemical element? |
The symbol of an element is a one- or two-letter abbreviation for its name. The first letter is always capitalized, the second is always lowercase. |
|
|
How do elements get their names? |
The people that discover an element get to name them. Names can come from Greek, Latin, countries, continents, their English name, or a famous scientist. |
|

|
some common elements |
|
|
molecule |
a combination of two or more atoms that are chemically bound together in a specific shape; they can be atoms of the same element or different elements therefore they are not necessarily the same chemical compounds.
example: water is a compound and a molecule; hydrogen gas is a molecule but not a compound because the two atoms are the same element |
|
|
chemical formula |
uses numbers and symbols for the elements to indicate which elements are present in a substance and how many atoms of each element are present in that substance (like a recipe for a substance) |
|
|
subscript |
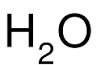
a number that is written below the symbol of an element in a chemical formula, such as the 2 in |

