![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
95 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is a use of alkenes? |
Polymerisation |
|
|
|
What is more healthy saturated or unsaturated fats? |
Unsaturated |
|
|
|
What is polymerisation? |
Joining monomers to make polymers |
|
|
|
What is a use of alkenes? |
Polymerisation |
|
|
|
Front (Term) What is electrolysis? |
Back (Definition) The breakdown of a substance using electricity It requires a liquid- electrolytes The free ions conduct electricity The electrons are taken away by the anode and given away by the cathode Turned back into atoms |
Cathode/ anode |
|
|
What is reduction? |
Back (Definition) Oxygen is removed |
|
|
|
Front (Term) In the reactivity series, metals higher than carbon have to be extracted by electrolysis True or false? |
Back (Definition) True |
|
|
|
What charge do atoms have overall? |
Neutral |
|
|
|
What charge do electrons have? |
Negative |
|
|
|
What does the mass number tell you? |
Number of protons and neutrons |
|
|
|
Why is the extraction of pure copper expensive? |
There are many stages and copper rich ores are on short supply |
|
|
|
How does an atom become an ion? |
If electrons are added or removed |
|
|
|
Which electrode do copper ions move toward? |
Cathode Negatively charged |
|
|
|
Why does aluminium react with iron oxide? |
Aluminium is more reactive |
|
|
|
What type of reaction releases energy from a fuel? |
Exothermic |
|
|
|
What is limestone mainly made from? |
Calcium carbonate |
|
|
|
How are earthquakes formed? |
Sudden movement Convection currents in the mantle Heat released by radioactive processes |
|
|
|
What charge do protons have? |
Positive |
|
|
|
What does the atomic number tell you? |
Number of protons |
|
|
|
What does the group number tell you? |
How many electrons are in the last outer shell |
|
|
|
What is ionic bonding always between? |
Metal and non metal |
|
|
|
Give two reasons why the percentage of carbon dioxide in the atmosphere has changed. Ignore human activities |
CO2 dissolved in oceans Used by plants Locked in fossil fuels |
|
|
|
What is calcium hydroxide used for? |
It is an alkali used to neutralise acidic soil |
|
|
|
How many electrons are in the first shell? |
2 |
|
|
|
What does thermal decomposition mean? |
When one substance chemically changes into at least 2 new substances when heated |
|
|
|
What are alkanes? |
Saturated hydrocarbons They have single carbon bonds |
|
|
|
What are the disadvantages of quarrying limestone? |
Destroys habitats Creates dust and noise pollution Produces tips |
|
|
|
What are the advantages of quarrying limestone? |
Improves local economies Strengthens roads Creates jobs |
|
|
|
What is an ore? |
An ore contains enough metal to make extraction economically worth while |
|
|
|
What is bioleaching? |
Bacteria get energy from the bond between two substances, thus separating them |
|
|
|
What is phytomining? |
Plants are grown in soil that contains metal The plants can't use or get rid of the copper so it builds up in the leaves The plant is burnt The metal is collected from the ash |
|
|
|
What are three common properties of metals? |
Malleable Conductor Durable |
|
|
|
What is an alloy? |
A mixture of two metals |
|
|
|
What is crude oil? |
A mixture of hydrocarbons |
|
|
|
Before fractional distillation, why is crude oil not useful? |
The different hydrocarbons have different properties- Boiling points Melting points Viscosity Etc... |
|
|
|
Are the hydrocarbons chemically bonded in crude oil? |
No |
|
|
|
What are the properties of short chained molecules? |
Very flammable Volatile (turns into a gas easily) Low viscosity (flows easily) |
|
|
|
How is crude oil separated? |
Fractional distillation -in the fractionating Column it I hottest at the bottom and coolest at the top The vaporised oil rises up and Condenses at fractions |
|
|
|
Where would a long chained molecule condense on a fractional distillation column? |
At the beginning- where it is hottest |
|
|
|
What is an alkane? |
The fractions of crude oil are hydrocarbons called alkanes |
|
|
|
Name four alkanes |
Methane ethane propane and butane |
|
|
|
Millions of years ago the Earth was covered in volcanoes. How did this help create oceans? |
The water vapour and steam condensed to form oceans |
|
|
|
Why is it difficult to dispose of waste rock? |
There are large amounts |
|
|
|
Why is it difficult to predict a volcanic eruption? |
Hard to monitor what is happening deep under the crust. |
|
|
|
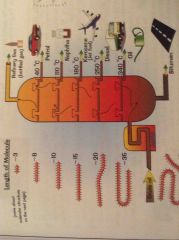
How is crude oil separated? |
Fractional distillation -in the fractionating Column it I hottest at the bottom and coolest at the top The vaporised oil rises up and Condenses at fractions |
|
|
|
What does hydrogenation do to vegetable oils? |
Hardens them; increases melting points; solid at room temp
Changes double bonds to single |
|
|
|
What are the conditions needed for hydrogenation? |
60 •c and a nickel catalyst |
|
|
|
What does limestone thermally decompose to? |
Calcium oxide and carbon dioxide |
|
|
|
What is the general formula for alkanes? |
Cn H 2n+2 |
|
|
|
What is the general formula for alkanes? |
Cn H 2n+2 |
|
|
|
If a fuel is burning and it releases sulfur dioxide what is the disadvantage for the environment? |
It produces acid rain |
|
|
|
What is a biofuel? |
They are fuels creates from renewable resources |
|
|
|
What does cracking do? |
It splits up long chained molecules |
|
|
|
What does cracking do? |
It splits up long chained molecules |
|
|
|
What type of reaction is cracking? |
Thermal decomposition |
|
|
|
What does cracking do? |
It splits up long chained molecules |
|
|
|
What type of reaction is cracking? |
Thermal decomposition |
|
|
|
Describe the process of cracking |
The long chained hydrocarbon is vaporised It is passed over a catalyst at a high temp of 400- 700•c |
|
|
|
What are the products of cracking? |
Alkane and alkene |
|
|
|
What are the products of cracking? |
Alkane and alkene |
|
|
|
What is the formula for alkenes? |
C3 H6 |
|
|
|
What are the products of cracking? |
Alkane and alkene |
|
|
|
What is the formula for alkenes? |
C3 H6 |
|
|
|
How do you test for an alkene? |
Bromine water It would de colourise because the double bond had opened up |
|
|
|
What are the products of cracking? |
Alkane and alkene |
|
|
|
What is the formula for alkenes? |
C3 H6 |
|
|
|
How do you test for an alkene? |
Bromine water It would de colourise because the double bond had opened up |
|
|
|
What does a alkene look like? |
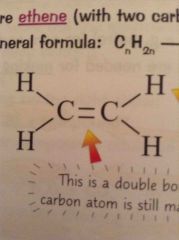
A double bond between two carbons and diagonal bonds |
|
|
|
What are the products of cracking? |
Alkane and alkene |
|
|
|
What is the formula for alkenes? |
C3 H6 |
|
|
|
How do you test for an alkene? |
Bromine water It would de colourise because the double bond had opened up |
|
|
|
What does a alkene look like? |
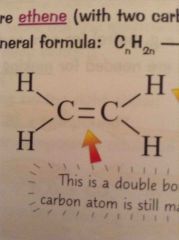
A double bond between two carbons and diagonal bonds |
|
|
|
How is ethanol made? |
By reacting steam with ethene |
|
|
|
What is a use of alkenes? |
Polymerisation |
|
|
|
What is polymerisation? |
Joining monomers to make polymers |
|
|
|
What would a polymer ethene substance be called? |
Polyethene |
|
|
|
What is a disadvantage of polymers? |
They do not biodegrade |
|
|
|
Describe the process of cold pressing. |
The seeds are crushed below 40•c, distillation removes water and impurities |
Plant oils |
|
|
What is more healthy saturated or unsaturated fats? |
Unsaturated |
|
|
|
Where on tectonic plates do earthquakes and volcanoes occur? |
On the boundary between two tectonic plates |
|
|
|
How did the atmosphere evolve? |
1-volcanoes gave out gases 2-green plants evolved and produced oxygen 3-the ozone layer was formed-blocking out harmful rays from the sun |
|
|
|
Why are ultrasound waves used to look deep under ground? |
They can pick up the different structures on the earth |
|
|
|
What was the Miller-Urey experiment? |
It simulated the early atmosphere to see how life was formed and a stable atmosphere |
|
|
|
What was the product of the Miller-Urey experiment? |
Amino acids |
|
|
|
What is an emulsion? |
Lots of droplets suspended in another liquid |
|
|
|
What is an example of where emulsions are used in our lives and why do the properties make it effective? |
Salad dressings Shaking olive oil and vinegar together makes an emulsion that coats the salad better |
|
|
|
What is the structure of the earth? |
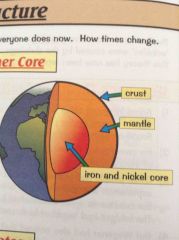
|
|
|
|
What are the parts of an emulsifier? |
Hydrophobic tail-likes oil hates water Hydrophilic head-likes water hates oil |
|
|
|
What is an advantage of using emulsifiers? |
It gives products a longer shelf life as they do not spread out It produces food with lower fat |
|
|
|
What is a disadvantage of emulsifiers? |
It can have allergies |
|
|
|
What was Wegener's theory of continental drift? |
Fossils of very similar plants and animals were found on opposite sides of the Atlantic Ocean The coastlines of Africa and South America matched like a jigsaw The rock layers matched |
|
|
|
What is the structure of the earth? |
|
|
|
|
Why do tectonic plates move? |
The convection currents in the mantle causes the plates to drift |
|
|
|
Describe how to decrease the percentage of of unsaturated fat in olive oil. (3marks) |
Hydrogenation Olive oil is reacted with hydrogen Nickel catalyst Temperature of 60•c |
|
|
|
Describe a test to show that carbon dioxide is produced. Give the result. (2marks) |
Limewater Turns cloudy |
|

