![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
Group 1- features |
Includes: lithium etc Have 1 outer electron, so are very reactive as they can easily form a 1+ ion with a stable electronic structure. As you go down they become more reactive as the one electron is further from the nucleus. All have low melting and boiling points. Very soft Reactions with cold water produces hydroxide and hydrogen gas. All react vigorously- reactivity increases down the group because the outer electron is lost more easily in the reaction. Lithium will fizz furiously, sodium and potassium do the same but also met in heat of reaction. |
|
|
Group 0 (noble gases) features |
Last row- helium etc All colourless gases at room temperature All monatomic- means their gases are made up from single atoms (not molecules) Are inert due to a full outer shell Non-flammable These properties make them hard to be discovered Uses include: argon- lightbulbs/ argon and helium- protects metals that are being welded/ helium- airships and party balloons. Melting/boiling points and density all decrease as you move dowb |
|
|
Reaction rates |
Rate of reaction = amount of reactant used or amount of product formed / time Experiments to follow rates of reactions: 1) precipitation- mix 2 reactant solutions on a flask and place on a cross in paper. Time how long the cross takes to disappear- shorter amount of time means faster rate of reaction. Subjective to person observing cross. 2) change in mass- use a mass balance to measure loss of mass. Ass gas escapes the lost mass is measured on scales. The quicker the mass drops the faster the reaction is. Reaction has finished when mass does not decrease anymore. 3) the volume of gases given off- use a gas syringe to measure volume of gas given off. The more gas given off during a set time interval, the faster the reaction. Need to be careful that you are using right size of syringe, if reaction is too vigorous than the plunger can be blown out of the end of the syringe. |
|
|
Rate experiments involving gases |
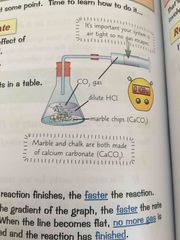
Experiment to measure how surface area affects rate- use marble chips and hcl. set up apparatus. Measure the volume of gas produced, take regular time intervals. Plot results OMG graph- Time on x, volume on y. Repeat but with powders chalk and same concentration and Colin of hcl. A steeper graduants means a faster rate of reaction- so using finer particles means a faster rate of reaction as it has a larger surface area. A higher concentration of acid also causes a faster rate of reaction. |
|
|
Rate experiments involving precipitation |
A higher temperature means a higher rate of reaction as the particle have more energy so collide more often. You can see this when reacting sodium thiosulfate and hydrochloride acid in the cross experiment. |
|
|
Calculating rates |
If the graph is in a straight line: fine 2 points and make triangle from the 2 points, than find values. Next find gradient. Height = change in y Base = change in x Gradient = change in y/ change in x. If graph is curved draw a tangent of a curve and repeat steps above |
|
|
Calculating rates |

If the graph is in a straight line: fine 2 points and make triangle from the 2 points, than find values. Next find gradient. Height = change in y Base = change in x Gradient = change in y/ change in x. If graph is curved draw a tangent of a curve and repeat steps above |
|
|
Collision theory |
Rates of reaction is explained by collision theory. The rate of a chemical reaction depends on: the collision frequency and the energy transferred during a collision (the minimum energy that particles need to react when they collide is called the activation energy. Increasing temperature increases rate as the particles have more energy so move faster and therefore collide more often. Increasing concentration (or pressure) increases rate. With a higher concentration there is more particles so they collide more often. Higher surface area means a higher rate. With more area to collide with, there are more collisions so rate increases. |
|
|
Catalysts |

Catalysts increase the rate of reaction without being chemically changed or used in the reaction. They work by decreasing the activation energy needed for a reaction to occur. They do this by providing an alternative pathway that has a lower activation energy. As a result more of the particles have at least the minimum amount of energy needed. |
|
|
Endothermic and exothermic reactions |
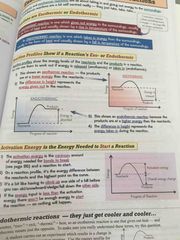
Exothermic gives out energy. The product in an exothermic reaction are at a lower energy than the reactants. Endothermic takes in energy. The products have a higher energy level than the reactants. The activation energy is the minimum amount of energy needed for bonds to break. If the energy input is less than the activation energy nothing will happen. |
|
|
Measuring temperature changes |
To measure tenpteeture change, complete experiment in a polystyrene cup in a large beaker of cotton wool (the cotton wool gives insulation to help limit energy transfer to or from reaction mixture). Add reactants than add lid with small hole for the Thermometer. The change in temperature depends on the reagents used. DISSOLVING SALTS IN WATER: dissolving ammonium chloride decreases temperature (endothermic). Dissolving calcium chloride increases temperature (exothermic). NEUTRALISATION: mostly exothermic however reaction between ethanoic acid and sodium carbonate is endothermic. DISPLACEMENT: in this reaction a more reactive element displaces a less reactive element in a compound- which releases energy (exothermic) PRECIPITATION: are exothermic. |
|
|
Bond energies |
Breaking bonds is endothermic. Bond forming is exothermic. Energy must be supplied to break bonds and energy is released when bonds are formed. To calculate energy changes and therefore wether bonds are broken or formed: see picture |

