![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
56 Cards in this Set
- Front
- Back
|
What are ions |
Ions are charged particles they can be single atoms or groups of atoms |
|
|
Why do atoms gain or lose electrons |
To become stable |
|
|
What happens when metals form ions |
They lose electrons from their outer shell to form positive ions |
|
|
What happens when non metals form ions |
They gain electrons into their outer shell to form negative ions |
|
|
What happens when non metals form ions |
They gain electrons into their outer shell to form negative ions |
|
|
If 2 electrons are lost what is the ions charge |
2+ |
|
|
What happens when non metals form ions |
They gain electrons into their outer shell to form negative ions |
|
|
If 2 electrons are lost what is the ions charge |
2+ |
|
|
If 3 electrons are gained what is the charge on the ion |
3- |
|
|
What happens when non metals form ions |
They gain electrons into their outer shell to form negative ions |
|
|
If 2 electrons are lost what is the ions charge |
2+ |
|
|
If 3 electrons are gained what is the charge on the ion |
3- |
|
|
What happens when a metal and non metal react |
The metal atom loses electrons to become a positively charged ion the non metal gains these electrons to form a negatively charged ion |
|
|
What happens when non metals form ions |
They gain electrons into their outer shell to form negative ions |
|
|
If 2 electrons are lost what is the ions charge |
2+ |
|
|
If 3 electrons are gained what is the charge on the ion |
3- |
|
|
What happens when a metal and non metal react |
The metal atom loses electrons to become a positively charged ion the non metal gains these electrons to form a negatively charged ion |
|
|
What is ionic bonding |
When oppositely charged ions are strongly attracted by electrostatic forces |
|
|
What happens when non metals form ions |
They gain electrons into their outer shell to form negative ions |
|
|
If 2 electrons are lost what is the ions charge |
2+ |
|
|
If 3 electrons are gained what is the charge on the ion |
3- |
|
|
What happens when a metal and non metal react |
The metal atom loses electrons to become a positively charged ion the non metal gains these electrons to form a negatively charged ion |
|
|
What is ionic bonding |
When oppositely charged ions are strongly attracted by electrostatic forces |
|
|
What groups are elements most likely to form ions in |
1 , 2 , 6 ,7 |
|
|
What ions do group 1 and elements form |
They are metals that lose electrons to form positive ions (cations) |
|
|
What ions do group 1 and elements form |
They are metals that lose electrons to form positive ions (cations) |
|
|
What type of ion do elements in group 6 and 7 form |
They are non metals that gain electrons to form negative ions (anions) |
|
|
What ions do group 1 and elements form |
They are metals that lose electrons to form positive ions (cations) |
|
|
What type of ion do elements in group 6 and 7 form |
They are non metals that gain electrons to form negative ions (anions) |
|
|
Why do elements in the same group form ions with the same charge |
They all have same number of outer electrons so they lose or gain the same number to get an outer shell |
|
|
What ions do group 1 and elements form |
They are metals that lose electrons to form positive ions (cations) |
|
|
What type of ion do elements in group 6 and 7 form |
They are non metals that gain electrons to form negative ions (anions) |
|
|
Why do elements in the same group form ions with the same charge |
They all have same number of outer electrons so they lose or gain the same number to get an outer shell |
|
|
Ionic equation for sodium becoming an ion |
Na-> Na+ + e- |
|
|
What ions do group 1 and elements form |
They are metals that lose electrons to form positive ions (cations) |
|
|
What type of ion do elements in group 6 and 7 form |
They are non metals that gain electrons to form negative ions (anions) |
|
|
Why do elements in the same group form ions with the same charge |
They all have same number of outer electrons so they lose or gain the same number to get an outer shell |
|
|
Ionic equation for sodium becoming an ion |
Na-> Na+ + e- |
|
|
Ionic equation of magnesium becoming and ion |
Mg ---> mg2+ + 2e- |
|
|
What ions do group 1 and elements form |
They are metals that lose electrons to form positive ions (cations) |
|
|
What type of ion do elements in group 6 and 7 form |
They are non metals that gain electrons to form negative ions (anions) |
|
|
Why do elements in the same group form ions with the same charge |
They all have same number of outer electrons so they lose or gain the same number to get an outer shell |
|
|
Ionic equation for sodium becoming an ion |
Na-> Na+ + e- |
|
|
Ionic equation of magnesium becoming and ion |
Mg ---> mg2+ + 2e- |
|
|
Ionic equation for chlorine becoming an ion |
Cl+e- ---> Cl- |
|
|
What ions do group 1 and elements form |
They are metals that lose electrons to form positive ions (cations) |
|
|
What type of ion do elements in group 6 and 7 form |
They are non metals that gain electrons to form negative ions (anions) |
|
|
Why do elements in the same group form ions with the same charge |
They all have same number of outer electrons so they lose or gain the same number to get an outer shell |
|
|
Ionic equation for sodium becoming an ion |
Na-> Na+ + e- |
|
|
Ionic equation of magnesium becoming and ion |
Mg ---> mg2+ + 2e- |
|
|
Ionic equation for chlorine becoming an ion |
Cl+e- ---> Cl- |
|
|
Ionic equation for oxygen becoming an ion |
O + 2e- ----> O2- |
|
|
NaCl dot and cross diagram |
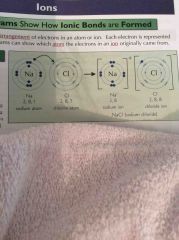
|
|
|
Dot and cross diagram for magnesium oxide |

|
|
|
MgCl2 |

Back (Definition) |
|
|
Pros and cons of dot and cross diagrams |
Pros: Show how ionic compounds form Cons: Don't show structure of compounds How they are arranged The size of ions |

