![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
32 Cards in this Set
- Front
- Back
|
Catalyst in making high density polyethene |
Ziegler natta |
|
|
Conditions for making HDPE |
Normal atm Temp is 60 Catalyst Ziegler natta |
|
|
Conditions for making LDPE |
High pressure 1200atm 200 degrees Catalyst is traces of oxygen Exothermic so water is added to dissipate heat |
|
|
What does branching do to LDPE |
Stops close packing So lover VDw forces Results in lower softening temp Quite flexible Stretches well Fairly low density |
|
|
Uses of LDPE |
Plastic bags Sheeting Insulation |
|
|
Due to no or little branching in chain what does that mean for HDPE |
Close packaging Strong VDW Higher softening temp Described as highly crystalline Higher density |
|
|
Uses of LDPE |
Buckets Bottles |
|
|
Uses of polypropene |
Synthetic carpets |
|
|
Uses of PvC |
Window frames. Guttering. Rain gear |
|
|
Polystyrene uses |
Insulating cups |
|
|
What does non-biodegrade mean |
Cannot be broken down by micro-organisms |
|
|
Monomer for polystyrene |
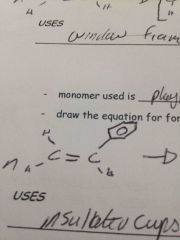
Back (Definition) |
|
|
Monomers for PeT |
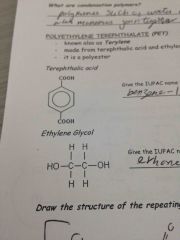
Back (Definition) |
|
|
Monomers for nylon |

Back (Definition) |
|
|
Uses for nylon |
Nylon tights. Sports clothing |
|
|
What is a polymer |
A large molecule made of many smaller ones |
|
|
Structure of glycine |
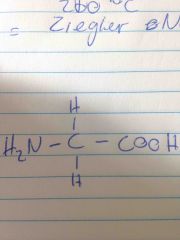
Back (Definition) |
|
|
Structure of alanine |
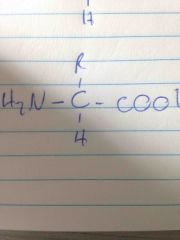
Back (Definition) |
|
|
Why does lysine have a high Mp |
Due to Zwitter ions and strong attractions between molecules |
|
|
How do enzymes work? |
They have an active site. Lock and key mechanism Lowers activation energy |
|
|
How many electrons are there in the delocalises Pi electron system in a benzene ring |
6 |
|
|
What is the anti cancer drug (draw) |
Back (Definition) |
|
|
What is reagent C |
NaNo2 |
|
|
What does a low Rf value indicate |
It is more soluble in stationary phase than mobile phase |
|
|
Formula for chrome alum |
K2SO4.Cr2(SO4)3.24H2O |
|
|
Explain why it is nessesary to keep temp below 10 degrees in making a diazonium ion |
As it decomposes above 10 degrees |
|
|
Explain peak integrations |
Ratio of x:y:z State molecule it applies to E.g ch3:ch2:ch3 |
|
|
What turns CH3CONH2 into CH2CN |
p4O10 |
|
|
How does chemisorption take place |
Molecules absorbed. Weakens bonds. Lowers activation energy |
|
|
Role of Hb |
Oxygen forms bond with Fe2+ carried around body c |
|
|
What is the anti cancer drug (draw) |
Back (Definition) |
|
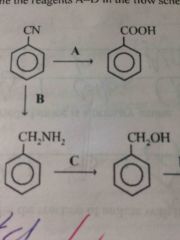
What is reagent C |
NaNo2 |

