![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
370 Cards in this Set
- Front
- Back
|
What is mass?
|
Refers to the amount of matter in a sample
|
|
|
What is matter?
|
Anything that occupies space and has mass
|
|
|
what is volume?
|
how much room a sample takes up in space
|
|
|
Density?
|
the ratio of an object's mass to volume: mass/volume (g/mL)
|
|
|
Pressure?
|
the force that a sample of gas in a closed container exerts on the walls
|
|
|
what are pressures units and how do they compare to each other?
|
760 torr = 760mmHg = 1 atm = 101.3kPa
|
|
|
Which instruments measure pressure?
|
barometer and manometer
|
|
|
What is a barometer?
|
measures pressure using liquid mercury
|
|
|
What is a manometer?
|
measures pressure using liquid mercury
|
|
|
What is energy? what are its units?
|
the ability to do work or transfer heat.
calories and joules |
|
|
what is a calorimeter
|
measures energy?
|
|
|
what is heat?
|
the flow of energy from a higher temperature object to a lower temperature object.
|
|
|
what is heat content?
|
heat.
therefore an increase in heat content is an increase in heat energy NOT temperature |
|
|
what is temperature?
|
measures the average kinetic energy of molecules in a sample; as the molecules move faster the temperature increases
|
|
|
What is heat capacity?
|
the amount of heat something must absorb for its temperature to be raised 1 degree Celsius.
|
|
|
What's the heat capacity equation?
|
q(heat)=mass(specific heat)(delta T)
|
|
|
What is Kelvin in Celsius?
|
-273
e.g. 273K is 0 degrees Celsius |
|
|
What is an atom?
|
the smallest particle of an element that still retains the chemical properties of that element.
|
|
|
what is a nucleon?
|
a proton or neutron; they both reside in the nucleus.
|
|
|
what is a positively charged ion called?
|
cation
|
|
|
what is a negatively charged ion called?
|
anion
|
|
|
what is an element? how do atoms relate to an element?
|
the most fundamental unit of matter, they cannot be broken down without losing their identity
an atom is the smallest bit of an element |
|
|
What are the vertical columns called on the periodic table?
|
groups
|
|
|
what are the horizontal columns called on the periodic table?
|
periods
|
|
|
What is the atomic number?
|
number of protons in nucleus; identity of element
|
|
|
what is the mass number made up of?
|
sum of mass of neutrons + protons (both have mass of approximately 1 amu)
|
|
|
whats an isotope?
|
elements that differ in the number of neutrons in its nucleus (same protons)
therefore they have different masses |
|
|
what is the atomic weight of an element?
|
an average of mass numbers representing the combination of isotopes on earth
|
|
|
What is a diatomic molecule?
|
when a molecule consists of 2 atoms (DONT HAVE TO BE THE SAME)
e.g. although O2 is a diatomic molecule, HCL is also a diatomic molecule |
|
|
What are the 7 elements that exist diatomically?
|
H2, O2, Br2, F2, I2, N2, Cl2
|
|
|
What is the weight of H2SO4?
|
98 amu
|
|
|
What is the empirical formula?
|
shows the ratios of atoms within a molecule, but not the EXACT amount of atoms in a given molecule.
|
|
|
what is the empirical formula of C2H6?
|
CH3...
find largest whole number that will divide in all subscripts. In this case 2. |
|
|
what is percent composition?
|
percent of a substance contained within a molecule in terms of mass.
|
|
|
What is percent composition of O in H2O2?
|
94%...
Total weight: 34 amu weight of O: 32 amu 32amu/34amu = 94% |
|
|
What is avrogados number? and what is its significance?
|
6.02 x 10^23...
that many amu in a mole. |
|
|
75% mercury by mass
25% chlorine by mass find empirical formula |
HgCl2...
assume 100g 75g Hg 25g Cl /200g/mol /35g/mol =0.375mol =0.700mol ratio is 1:2 therefore HgCl2 |
|
|
What about converting to molecular formula?
|
???? EDIT
|
|
|
NH3 (g) + O2 (g) --> NO (g) + H2O (l)
Balance this. |
4NH3 (g) + 5O2 (g) --> 4NO (g) + 6H2O (l)
|
|
|
4NH3 (g) + 5O2 (g) --> 4NO (g) + 6H2O (l)
if 34g of ammonia and 32g of oxygen are coalesced how many grams of nitrogen will be produced? |
0.8 moles
or 24g |
|
|
Which are more stable, low energy or high energy states?
|
low energy
|
|
|
What does the universe prefer in terms of entropy and enthalpy? how does this relate to stability?
|
high entropy, low enthalpy
because > entropy and < enthalpy mean more stability. |
|
|
What is the entropy, and what variable represents it?
|
the amount of disorder.
The variable is S. if a reaction's delta S is positive, the reaction gained entropy, and vice versa. |
|
|
what is enthalpy? how is it symbolized?
|
enthalpy is the energy states of reactants or products, how much energy they hold. It is symbolized by H.
|
|
|
What is an exothermic reaction?
What is an endothermic reaction? |
exothermic = energy released, Δ H < 0, less enthalpy
endothermic = energy absorbed, Δ H > 0, higher enthalpy |
|
|
What is heat of formation? what's its symbol?
|
heat of formation is the amount of heat that's released or absorbed when 1 mole of compound is formed from its elements. It's symbol is Δ Hf
|
|
|
What is the heat of formation of gaseous carbon dioxide:
CO2C (s) + O2 (g) --> CO2 (g) delta H = -393 kJ/mol |
heat is released during the reaction; exothermic. When 1 mole of CO2 (g) is formed, 393kJ of energy is released.
|
|
|
What is the heat formation of elemental atoms or molcules? e.g. C, Ni, N2, H2
|
For all elements, the heat of formation is zero.
|
|
|
What's the heat formation equation?
|
Δ Hf = Δ Hf (products) - Δ Hf (reactants)
|
|
|
C6H12O6(s) + 6O2 (g) ---> 6CO2 (g) + 6H2O (l)
heat of formations: C6H12O6 (s) = -1273kJ/mol CO2 (g)= -393kJ/mol H2O (l)= -286kJ/mol |
-4074 kJ - (-1273kJ) = - 2801 kJ
therefore reaction is exothermic |
|
|
What is Hess's Law?
|
if a reaction is carried out in a series of steps, Δ H for the reaction will be equal to the sum of enthalpy changes for the individual steps. Doesn't matter how many steps in between.
|
|
|
What is a spontaneous reaction?
|
one that occurs at a given temperature without the input of energy.
|
|
|
Can endothermic reactions occur spontaneously?
|
yes, if the increase in enthalpy is made up for a greater increase in entropy. vice versa for exothermic reactions.
|
|
|
What determines of a reaction will or won't occur spontaneously?
|
Gibbs free energy:
ΔG = ΔH - TΔS (temperature measured in K) |
|
|
What if the Δ G for reaction is negative? positive? zero?
|
negative: reaction occurs spontaneously in the forward direction.
positive: reaction occurs spontaneously in the reverse direction zero: the reaction is in equilibrium |
|
|
How do electrons exist?
|
they exist in orbitals.
|
|
|
What is an orbital?
|
an orbital is just an area where an electron may be found, they come in a variety of shapes and sizes. It is also called a "probability function"
|
|
|
What is called a probability function?
|
an orbital. Or the likelihood that an electron will be found in a particular location.
|
|
|
What are the four significant shapes called?
|
s, p, d (n-1) , f(n-2)
|
|
|
what is the Heisenberg principle?
|
one cannot know an electrons position and momentum at the same time.
|
|
|
What is de broglies hypothesis?
|
matter can be thought of as having properties of both a particle and a wave.
|
|
|
What is the bohr model?
|
the incorrect idea that electrons orbit the nucleus in true orbits, like planets orbit the sun.
|
|
|
where on the periodic table are the different subshells located?
|
s = group 1A, 2A, + helium
d(n-1) = B groups p = 3A - 8A groups f (n-2) = detached elements |
|
|
What is the electron configuration?
|
how electrons of an atom are arranged.
|
|
|
How would the electron configuration change from a F atom to a F - ion?
|
add 1 electron to the subshells.
therefore 1s^2 2s^2 2p^5 becomes: 1s^2 2s^2 2p^6 (same as neon atom) |
|
|
What is significant with f sub shell?
|
if an element has an atomic number greater than 57 some of its electrons are in the f subshell, or are in f orbitals. So terbium (Tb) would have some electrons in f orbitals.
|
|
|
what is the electron configuration for Tc?
|
1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 5s^2 4d^5
|
|
|
What is the Aufbau principle?
|
states that electron sub shells are completely filled before being placed in the higher one.
|
|
|
Which elements violate the Aufbau principle and in what way?
|
Copper and Chromium promote a 4s electron to the 3d orbital.
|
|
|
What in an inert gas?
|
noble gas, stable octet of electrons
|
|
|
What are valence electrons?
|
electrons in the outermost energy shell.
|
|
|
What are the # subshells representing?
|
They represent the "orbits" (use this analogy for a better picture)
so 1s^2 is the first two electrons in the first "orbit" 2s^2 + 2p^6 = 8 electrons in 2nd "orbit" etc. |
|
|
what is atomic theory?
|
In chemistry and physics, atomic theory is a theory of the nature of matter, which states that matter is composed of discrete units called atoms, as opposed to the obsolete notion that matter could be divided into any arbitrarily small quantity.
|
|
|
what is quantum theory?
|
A theory developed in early 20th century, according to which nuclear and radiation phenomena can be explained by assuming that energy only occurs in discrete amounts called quanta.
|
|
|
What is radioactivity?
|
when an unstable nucleus undergoes nuclear decay.
|
|
|
What is used to detect and measure radioactive particles?
|
Geiger counter
|
|
|
What is alpha decay?
|
when a nucleus gives off an alpha particle and its atomic number is reduced by 2, its mass number reduced by 4.
|
|
|
In alpha decay, the alpha particle is a ___?
|
helium - 4 nucleus or 4/2 He.
|
|
|
Alpha particle emitted.
Answer this: 226/88 Ra ----> ??? |
----> 222/86 Rn + 4/2 He
(Notice Ra is now Rn due to change of atomic number to 86) |
|
|
What is beta (β) decay?
|
emission of a beta particle (β)
|
|
|
What is a β particle?
|
β particle is an electron "0/-1 e"
|
|
|
What must be done for beta decay to occur?
|
a neutron within the nucleus must be converted into a proton as seen in example (top number [mass number] does not change after conversion because protons and neutrons weigh same)
|
|
|
what is positron emission?
|
a positron is emitted.
|
|
|
What is a positron? what is it symbolized as?
|
positively charged particle, but it isn't a proton. symbol is 0/1 e.
|
|
|
What happens in positron emission in regards to neutrons/protons?
|
Proton converted to neutron.
|
|
|
what is gamma decay/radiation?
|
gamma ray emitted
gamma rays have neither mass nor charge and is represented as "0/0 γ" |
|
|
Why does gamma radiation occur?
|
release excess energy in conjunction with alpha, beta and positron
|
|
|
What is problem with nucleus that causes alpha decay?
|
nucleus too heavy
|
|
|
What is problem with nucleus that causes beta decay?
|
too many neutrons, too few protons
|
|
|
What is problem with nucleus that causes positron emission?
|
too many protons, too few neutrons
|
|
|
What is problem with nucleus that causes gamma decay?
|
too much energy in nucleus
|
|
|
What is half life?
|
time it takes for a substance to become half of the original amount.
|
|
|
If 100g sample half life is 1 year, and you leave sample for 3 years, what how much will be left?
|
12.5g
|
|
|
How do elements in the same period relate?
|
they have electrons in the same energy shells
|
|
|
How do elements in the same group relate?
|
they tend to have similar chemical and physical properties.
|
|
|
What is a family?
|
a family is a collection of elements from the same vertical group that have similar chemical properties (alkali, alkaline earth, noble gases)
|
|
|
Where are alkali metals located.. what group? what characteristics do they have?
|
group I
-extremely reactive -shiny grayish white metals -lower densities, lower melting points than other metals |
|
|
Where are alkaline earth metals located.. what group? what characteristics do they have?
|
group II
-reactive, not as much as alkali, but moreso than other metals like copper -look a lot like alkali metals |
|
|
What are active metals?
|
alkali and alkaline earth metals;
since they have similar qualities |
|
|
Where are halogens located.. what group? what characteristics do they have?
|
group VII
-very reactive, moreso than alkaline earth -qualities differ |
|
|
What is a metal? What characteristics do they have?
|
tend to give up electrons when they bond
-shiny -good conductors -malleable, ductile |
|
|
What is a non metal? What characteristics do they have?
|
tend to gain electrons when they form bonds
-poor conductors -brittle -low melting point |
|
|
What is a semimetal? What characteristics do they have?
|
can either gain or lose electrons in a bond
-possess characteristics of metals and non metals |
|
|
What is another name for a semi metal?
|
metalloid
|
|
|
What is an active metal?
|
-reactive metals that are in the s area
|
|
|
what is a transition metal?
|
all metals not in s area
-less reactive -harder -higher melting points |
|
|
COMPOUNDS that contain a transition metal are usually ___
|
they are usually intensely colored when in compounds
|
|
|
Where are metalloids on periodic table?
|
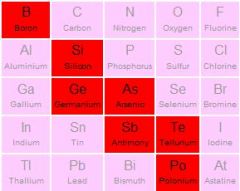
|
|
|
What is ionization energy?
|
energy required to remove an electron from an atom
|
|
|
What is the periodic behavior of ionization energy?
|
increase across the table; decreases down the table
|
|
|
What is electronegativity?
|
how much an atom "pulls" on electrons in a bond
|
|
|
What is the periodic behavior of electronegativity?
|
increase across the table; decreases down the table
|
|
|
What is atomic radius?
|
distance from the center of atom to the edge
|
|
|
What is the periodic behavior of atomic radius?
|
decreases across the table; increases down the table
|
|
|
What is metallic character?
|
how easily an atom gives up an electron in a bond
|
|
|
What is the periodic behavior of metallic character?
|
decreases across the table; increases down the table
|
|
|
What is lattice energy? what are units of it?
|
-binding energy of ionic solid
-measure of energy required to completely separate a mole of solid ionic compound into separate ions |
|
|
In ionic bonds, what does the electronegativity difference look like?
|
They differ substantially.
|
|
|
Why are ionic bonds so strong?
|
Electrostatic force or electrostatic attraction
|
|
|
What is an ionic bond?
|
metal & non metal
|
|
|
What are characteristics of ionic bonds?
|
solid at room temperature
-hard -brittle -high melting points -cannot conduct electricity (ions too close together) |
|
|
What is a covalent bond? when do they occur?
|
two non metals bond together.
-they occur when electronegativities are similiar (not big difference) |
|
|
Why do polar covalent bonds occur?
|
One atom has a greater electronegativity than the other in a molecule, causing it to hog electrons.
|
|
|
Describe H2O in terms of polar covalent bond.
|
O atom has large electronegativity, therefore partial negative charge.
H atoms have partial positive charge. |
|
|
What is a metallic bond? What is peculiar about them?
|
when two metals bond.
-all the valence electrons move freely in the sample, producing an attractive force that keeps the metal cations in place |
|
|
What is significant about the sea of mobile electrons in metallic bonds?
|
allows metallic bonds to conduct electricity and heat.
|
|
|
What is a single bond? Double bond? triple bond?
|
sharing one, two, and three pairs of electrons respectively.
|
|
|
What is bond energy?
|
the amount of energy it takes to break a bond
|
|
|
What is ΔH for reaction:
H2 + Br2 ----> 2HBr H-H bond: 436 kJ/mol Br-Br bond: 193 kJ/mol H-Br bond: 366 kJ/mol |
products= 732kJ
reactants= 629 kJ difference = -103 kJ (r-p) reaction should release 103 kJ of energy |
|
|
What shape is CCl4?
|
tetrahedral
|
|
|
What are characteristics of tetrahedral?
|
-109.5 degrees between
-no lone pairs, 4 electron pair sites (CCl4) |
|
|
What are characteristics of trigonal pyramidal?
|
-107 degrees
-1 lone pair, 4 electron pair sites (NH3) |
|
|
What are characteristics of bent?
|
-105 degrees
-2 lone pairs, 4 electron pair sites (H2O) OR 116 degrees -1 lone pair, 3 electron pair sites (SO2) |
|
|
What are characteristics of trigonal planar?
|
-120 degrees
-3 electron pair sites, no lone pairs (NO3) |
|
|
What are characteristics of linear?
|
-180 degrees
-no lone pairs, 2 electron pair sites CO2 |
|
|
What is special about Be (beryllium)?
|
it is stable with 4 valence electrons
|
|
|
What is special about boron (B) atoms?
|
stable with 6 valence electrons
|
|
|
When can polar bonds become non polar?
|
when the molecule is exactly symmetrical.
|
|
|
What are intermolecular forces?
|
Forces between different molecules
|
|
|
what are intramolecular forces?
|
Forces between atoms in a molecule.
|
|
|
What are characteristics of real gases?
|
-attract/repel each other
-occupy volume |
|
|
What are characteristics of ideal gases?
|
The theoretical view of gases as not occupying volume or attracting/repelling each other
|
|
|
If we have a 3L sample of gas at 200K and a pressure of 900 torr and change the temperature to 400K what will happen to the pressure? (if volume remains constant)
|
Pressure doubles to 1800 torr. molecules move much faster creating double pressure.
|
|
|
What is the kinetic molecular theory?
|
kinetic energy of a gas molecule increases proportionally with temperature in degrees Kelvin; particles are constantly moving and they move quicker at higher temperatures
|
|
|
If we start with a 3L sample at 200K and 900 torr, and increase volume to 6L what happens to the pressure?
|
It will become half. Becomes 450 torr. molecules have twice as much area to move around in.
|
|
|
What is the ideal gas equation? What's a good way to look at it?
|
PV=nRT
Remember T is Kelvin! values on same side are inversely proportional -values on different sides are directly proportional (when all other values are constant) |
|
|
What is the ideal gas constant?
|
R ate pi!
8.314 |
|
|
What is STP?
|
273K and 101.3kPA (1 atm, 760torr, 760 mmHg)
|
|
|
1 mole of gas at STP occupies:
|
22.4L of volume!
|
|
|
What is the formula for partial pressure?
|
Moles of gas A/Total moles in container = Partial pressure gas A/Total pressure of container
|
|
|
20 moles O, 30 moles H, 50 moles N. Total pressure is 500 torr. What are the partial pressures?
|
O -100 torr
H -150 torr N -250 torr |
|
|
How do you find partial volume of a gas?
|
Find moles
Multiply by 22.4L/mol (@ STP) |
|
|
How does hydrogen bonding work?
|
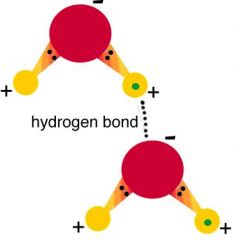
negative oxygen atoms are attracted to other H2O molecule positive hydrogen atoms. It's an intermolecular force.
|
|
|
What do changes of state involve?
|
they involves intermolecular forces, not covalent bonds.
|
|
|
What is an ionic bond crystal? What are characteristics? What is electrical conductivity?
|
non metal, metal.
-electrostatic attraction -high melting point -hard, brittle -only conductive electrically if molten or aqueous solution |
|
|
What is a covalent network? What are characteristics?
|
-One giant covalent bond
-Very high melting point -very hard -no electrical conductivity -e.g. diamond |
|
|
What is molecular crystals? Characteristics?
|
H-bonding, dipole-dipole, dispersion forces
-Low melting point -soft hardness -no electrical conductivity -e.g. H20 |
|
|
What is a metallic bond? Characteristics?
|
-Electrostatic attraction between catioins and sea of electrons
-Cu (s) -variable melting point -Malleable -high (electrons) |
|
|
What are 4 types of crystalline solids?
|
Ionic, covalent network, molecular, and metallic
|
|
|
What is a hydrate?
|
An ionic substance in which water molecules bond to the ions in a fixed ratio, it’s the same as being an element.
|
|
|
What is an example of a state or phase?
|
Solid, liquid, gas, etc.
|
|
|
When water is at 0 degrees Celsius what state/phase is it in?
|
Can be a liquid OR solid.
|
|
|
What is sublimation?
|
Going from gas directly to a solid or a solid directly to a gas.
|
|
|
What is the heat of fusion?
|
Amount of energy (in cal/g) needed to just pass through its melting point (from solid to liquid)
|
|
|
What is heat of vaporization or heat of condensation?
|
Amount of energy (in cal/g) that must be added to move a substance from liquid phase to gaseous phase.
|
|
|
In a phase change diagram, what are the flat parts representing?
|
Areas where there is a phase change, and the heat of fusion or vaporization must accumulate before the phase change occurs.
|
|
|
What does higher pressure do to affect phase change?
|
Makes it harder for a solid to become a liquid, and a liquid to become a gas; therefore melting and boiling points are raised
|
|
|
What does a reduced pressure do to affect phase change?
|
Makes it easier for a solid to become a liquid, and a liquid to become a gas; therefore melting and boiling points are lowered.
|
|
|
How does higher pressure affect water (an exception)? How does lower pressure affect water?
|
Higher pressure lowers melting and boiling points, lower pressure increases melting and boiling points (with water), which is counter intuitive.
|
|
|
What is the triple point in a phase diagram?
|
The point where a substance can be liquid, solid, or gas.
|
|
|
What is vaporization?
|
Going from liquid to gas
|
|
|
What is evaporation?
|
Even if a liquid is below its boiling point, some of the molecules will have enough energy to escape into the gaseous phase.
|
|
|
What ALWAYS occurs in a closed system for a liquid as it pertains to evaporation?
|
The sample will always have a part of it evaporating (and then condensing), while another part will evaporate to replace it.
-A vapor pressure is created |
|
|
What occurs if a liquid is in an open system?
|
-little bit evaporates, then is blown away, little more, then is blown away
-eventually entire sample of liquid evaporates, even if below boiling point, although the lower the temperature the longer this process takes |
|
|
If two equal buckets are filled with water and then the other with gasoline, which will fully evaporate first? (Temperature below boiling point) What does this mean?
|
The gasoline will evaporate first because it has weaker intermolecular forces than water molecules. This means the gasoline has a higher vapor pressure AND it’s more volatile than water.
|
|
|
How does temperature affect vapor pressure?
|
> temperature = > vapor pressure
|
|
|
If a container is open to the environment, what happens in terms of atmospheric pressure?
|
Vapor pressure = atmospheric pressure
|
|
|
What does atmospheric pressure equal
|
Patm = vapor pressure + partial pressure of atmospheric molecules
|
|
|
When the pressure above a liquid is all vapor pressure, or when vapor pressure equals atmospheric pressure, what occurs? (atmospheric pressure is always constant)
|
Boiling (???)
|
|
|
How does molecular weight affect vapor pressure?
|
> molecular weight = < vapor pressure
|
|
|
What are factors affecting vapor pressure?
|
Temperature, molecular weight and intermolecular forces
|
|
|
More volatile means…
|
Liquid has higher vapor pressure
-evaporates more readily when temperature is below boiling point (compared to less volatile liquid) |
|
|
Which phase has the most potential energy?
|
Gas (solid is lowest)
|
|
|
Which phase has greatest entropy?
|
Gas (solid, lowest)
|
|
|
What justifies an increase in energy that makes a phase change spontaneous?
|
The increase in entropy is higher than increase in energy
|
|
|
In the case of melting if the temperature is above melting point, what happens?
|
Phase change is spontaneous
|
|
|
In the case of boiling, if the temperature is above boiling point, what happens?
|
Phase change is spontaneous.
|
|
|
What is molarity equation, and what are the units?
|
Molarity (M) = # of moles solute/ number of liters of SOLUTION
|
|
|
What is molality equation, and what are the units?
|
Molality (m) = number of moles of solute/ number of kilograms of SOLVENT
|
|
|
When is a solvent saturated with solute?
|
When no more solute can be dissolved in solvent.
|
|
|
What is another word for limit of solubility?
|
Saturation
|
|
|
What is the main rule of solubility?
|
Generally polar dissolve polar, and non-polar dissolve non-polar
|
|
|
As temperature increases how is solubility of solids changed?
|
Increased solubility
|
|
|
As temperature increases how is solubility of liquids changed?
|
Unchanged
|
|
|
As temperature increases how is solubility of gases changed?
|
Decreased solubility (gas molecules too hyperactive)
|
|
|
As pressure increases how is solubility of solids changed?
|
No change
|
|
|
As pressure increases how is solubility of gases changed?
|
Increased solubility.
|
|
|
What is another word for ionic solutions?
|
Electrolytic solutions
|
|
|
What do we call ions in ionic solutions?
|
Electrolytes
|
|
|
How would you dissociate the ions of CaBr2?
|
2 Br (-) + 1Ca(2+)
|
|
|
When a solute is dissolved in a liquid solvent what happens to freezing point, boiling point and vapor pressure of the solvent?
|
Boiling point increases
-freezing point decreases -vapor pressure decreases |
|
|
How do you determine boiling point elevation and freezing point depression of solvent? (When solute dissolves in solvent)
|
Equation delta T =kmi
m=molality i=number of dissolved particles (moles) k=constant that is different for all solvents |
|
|
If sucrose raises the boiling point of a solvent by 1 degree K, then how much would KCL raise the boiling point of the same solvent? If the sucrose lowered the freezing point by 2 degrees, how much would the KCL decrease it?
|
2 degrees K.
KCL dissociates into 2 ions making “i” equal 2. Sucrose does not dissociate, therefore “i” value is only 1, making KCL increase boiling point by twice as much. For freezing point, using the same logic, we get KCL decreasing it by 4 degrees. |
|
|
Name ALL 4 solubility rules!
|
1) Most silver, lead, and mercury salts are INSOLUBLE except their nitrates/perchlorates
2) Most hydroxides are INSOLUBLE except with alkali metals or barium 3) All nitrates and perchlorates (CLO4-) are SOLUBLE 4) All alkali metals and ammonium (NH4+) compounds are SOLUBLE |
|
|
What is kinetics?
|
the study of the rates of reactions; how fast reactants are converted into products
|
|
|
What is equilibrium?
|
the point in a chemical reaction where the concentration of all the reactants and products cease to change.
|
|
|
What is the reaction rate?
|
the rate at which reactants are converted into products.
|
|
|
What must happen for a chemical reaction to occur?
|
reactant molecules must collide with enough energy and orientation so:
-reactant bonds are broken -and so new bonds can form |
|
|
What is the activated complex?
|
Same as transition state.
-The point where the reactant bonds and product bonds are both "half bonds" -is an extremely unstable, high energy arrangement of atoms |
|
|
What is the transition state?
|
Same as activated complex.
-The point where the reactant bonds and product bonds are both "half bonds" -is an extremely unstable, high energy arrangement of atoms |
|
|
What is the process of a chemical reaction?
|
1) reactant molecules collide with enough energy/orientation
2) old bonds begin to break, new bonds begin to form: this stage is called activated complex or transition state 3) old bonds completely break, and new bonds are formed |
|
|
What factors affect reaction rate?
|
1) concentration of reactants
2) surface area of reactants 3) temperature 4) nature of reactants 5) catalysts |
|
|
How does concentration of reactants affect reaction rate?
|
greater concentration of reactants, faster the reaction
-cannot change concentration of liquid/solid reactants -with gases pressure can be increased to increase concentration of gas reactant -solutions can also change concentration -therefore if solution/gas is in a reaction then concentration can change reaction |
|
|
How does surface area of reactants affect reaction rate?
|
The greater the surface area of reactants, the greater number of collisions so faster reaction rate.
-ONLY liquids and solids can change surface area... (liquids spraying a mist, solids by grinding up) |
|
|
How does temperature affect reaction rate?
|
Temperature has the greatest effect on reaction rate.
-higher temperature means molecules move faster -rule of thumb, increase in 10 d. C, reaction rate doubles |
|
|
How does nature of reactants affect reaction rate?
|
-since bond breaking must occur for reactions to occur, then reactants with weaker bonds will react quicker than stronger intramolecular bond reactants
-reactions between dissolved ions tend to be faster because bond breaking has already occurred |
|
|
How do catalysts affect reaction rate?
|
-increases rate of reaction without being consumed by it
-they lower activation energy requirements for a reaction, increasing reaction rate |
|
|
What is the activation energy?
|
In chemical reactions, the activation energy is the minimum amount of energy that must be supplied for the activation complex to be formed.
|
|
|
what is activation complex?
|
energy barrier that must be overcome in order for reactants to react
|
|
|
What does a small activation energy barrier mean?
|
not as much energy needed to produce activated complex, therefore a greater percentage of reactant collisions are likely to create products, resulting in a higher rate of reaction
|
|
|
What are 3 qualities of catalysts?
|
-catalysts increase rate of reaction by lowering activation energy
-catalysts are not consumed by a reaction -catalysts do not change the equilibrium of a reaction |
|
|
What are the 3 factors affecting frequency of collision?
|
-concentration of reactants
-surface area of reactants -temperature |
|
|
what are the 3 factors affecting energy of collisions?
|
-temperature
-nature of reactants -catalysts |
|
|
What two main factors affect rate of reaction?
|
Frequency of molecular collisions
and energy of molecular collision |
|
|
What is dynamic equilibrium?
|
the point where the forward and reverse reaction rates are equal, which means that the concentration of products and reactants stays constant
|
|
|
What is the equilibrium expression?
e.g. aA + bB <--> cC + dD |
K(eq) = [C]^c[D]^d
__________ [A]^a[B]^b concentration of products, to the exponent of coefficient, divided by concentration of reactants to the exponent of coefficient |
|
|
What is important to remember about equilibrium expressions?
|
only gaseous/solution reactants are used, because their concentrations can be changed
|
|
|
what would be the equilibrium expression for this reaction:
Bf3 (g) + 3H2O (l) <---> 3HF (aq) + H3BO3 (aq) |
K(eq) = [HF]^3[H3BO3]
____________ [BF3] water is not included because it is not a gas or solution |
|
|
In a reaction where the K(eq) is 100, what will the concentrations look like of reactants and products?
|
more than 99% product, less than 1% reactant
|
|
|
If a reaction at equilibrium is 66% product and 33% reactant in terms of concentration, what is the K(eq)?
|
2.
Because 66/33 = 2! |
|
|
If product and reactant concentrations are about the same, then what will the K (eq) look like?
|
it'll be around 1.
|
|
|
K(eq) > 1, which is favored: reactants or products?
|
products or forward reaction
|
|
|
K(eq) < 1, which is favored: reactants or products?
|
reactants or reverse reaction
|
|
|
What happens to K(eq) when more of a reactant is added?
|
doesn't matter, K(eq) stays constant
|
|
|
How would equilibrium work with a liquid in a closed container?
|
phase change equilibrium would occur.
Some water molecules would gather enough energy to become a gas, then lose energy and become a liquid with other water molecules becoming gases and taking its place. The ratio of gas to liquid would stay constant once K(eq) is established. |
|
|
What is le chatelier's principle?
|
The idea that a system at equilibrium will respond to a stress placed upon it in such a manner as to partially offset that stress.
|
|
|
A + B <---> C + D
increase A, what happens to products? |
- both C & D increase
|
|
|
A + B <---> C + D
Increase C & D, what happens to reactants? |
A & B increase
|
|
|
A + B <---> C + D
Increase concentration of A, what happens to reactants & products? |
due to increase concentration of A, more collisions occur with B, therefore increase in C & D
-because no extra B is added, as it reacts with the extra A to form more C & D, there is less B than there was originally -There is more A than there was originally |
|
|
2A (g) + B (g) <---> C (g)
[A] = 2 M [B] = 6 M [C] = 8 M If we add more A, what could be possible concentrations of reactants and products? |
[M means molarity or g/mol]
use K(eq) = 8 M ________ [2 M]^2[6 M] K(eq)=8/24 = 1/3; this stays constant. Possibility: A = 3 M (increase) B = 4 M (decrease) C = 12M (increase) so K(eq) stays constant, |
|
|
What happens to K(eq)
when temperature changes? |
it will change
|
|
|
How does heat (temperature) affect equilibrium?
|
causes equilibrium shift
AND changes K(eq) [the only factor that changes K(eq)] |
|
|
H + I + heat <---> J + K
What happens if you increase the temperature in this reaction? |
Think of heat as if its a reactant, because in this reaction heat is consumed in the forward direction, and heat is released in reverse.
-so as temperature increases the equilibrium is pushed to the right |
|
|
Pressure affects reactions that have ___
|
one or more gases
|
|
|
What is the haber process?
|
process where ammonia is created.
N2 (g) + 3H2 (g) <----> 2NH3 |
|
|
consider the following reaction (haber process):
N2 (g) + 3H2 (g) <----> 2NH3 If volume is decreased, what will happen? |
-equilibrium pushes to the right
-equilibrium will push in direction that produces fewer moles of gas |
|
|
Hypothetical reaction (not realistic):
N2 (g) + 3CL2 (g) <---> 4NH3 (g) what happens if pressure is increased? |
nothing.
both sides have equal numbers of moles, therefore no change in equilibrium. |
|
|
How do catalysts affect equilibrium?
|
They don't, they might help reaction achieve equilibrium more quickly, but won't change equilibrium.
Kinetics is different from equilibrium!!! |
|
|
What is formula for solubility product constant and what is it used for?
|
K(sys/sp) = [A]^a[B]^b
Used to find how soluble an ionic solid is. |
|
|
smaller K(sp) means..
|
ionic solid is less soluble.
|
|
|
Ionic solid dissolving:
PbCl2 (s) <----> Pb ²+ (aq) + 2Cl − (aq) products are ions! what is the equilibrium expression? |
K(sp) = [Pb ²+][Cl −]²
|
|
|
What is autoionization?
|
the slight dissociation of water where some H2O becomes H + (aq) and OH- (aq).
-it is reversible, and exists at equilibrium |
|
|
What is the equilibrium expression for the ionization of water?
|
K(w) = [H+][OH-]
|
|
|
For pure water at 25 degrees C, [H+] & [OH-] are both 10^-7 M. What is the equilibrium expression? Will the constant change if more [H+] or [OH-] is added to solution?
|
K(w) = [H+][OH-]
K(w) = [10^-7 M][10^-7 M] =10^-14 M^2 If temperature stays the same, the constant will NOT change... remember k(eq) never change unless temperature changes. |
|
|
[H+] = 10^-7 M
then pH is? |
pH = -log(10^-7) = 7
|
|
|
How do you find the log(10) of a number?
|
ask yourself "what is this number's exponent when written as a base ten number (10 to some power)?"
|
|
|
What is the log(10) of 400?
|
= 10^2
therefore between 2 and 3. |
|
|
What is Arrhenius theory?
|
-acids produce H+ in aqueous solution.
-bases produce OH- in aqueous solution. |
|
|
What is Lewis theory on acids & bases?
|
Acids are electron acceptors in solution
-Bases are electron donors in solution |
|
|
Which theory is widely accepted today as it pertains to acids & bases?
|
Bronsted - Lowry Theory.
|
|
|
What is bronsted-Lowry theory?
|
-acids are proton donors
-bases are proton acceptors |
|
|
In bronsted-lowry theory what does a proton mean?
|
a H+ ion (aq)
-it reacts with water to produce a hydronium ion: H3O+ (aq) |
|
|
What are amphoteric molecules/ions?
How is HCO3 - (aq) an amphoteric molecule? |
-can donate OR accept H+ (aq) ions, depending on what is in the solution
HCO3 -(aq): in acidic solutions: HCO3 -(aq) + H+ (aq) ---> H2CO3 (aq) in basic solutions: HCO3 - (aq) +OH- (aq) ----> CO3 (2- aq) + H2O (l) |
|
|
What is a strong acid or base?
|
acids and bases that dissociate completely and stay dissociated. Dissociation is considered irreversible.
|
|
|
What are the strong acids?
|
Hi Nick Clint! for Supper: hydrochloric acid or hydrobromic acid?
|
|
|
What are the strong bases?
|
Group 1 hydroxides such as LiOH, NaOH, KOH
|
|
|
What is pH?
|
pH=-log[H+]
|
|
|
at 25 degrees Celsius, p[H+] + p[OH-] = ???
|
14
|
|
|
What is the pH of 1.0 M HNO3 (aq)?
|
0, because:
HNO3 (aq) ---> H+ (aq) + NO3 - (aq) 1.0 M 1.0 M since pH is the -log[H+]: pH = -log[H+] = -log(1.0M) =-log(10^0 M) =0 |
|
|
What is the pH of 1.0 M KOH (aq) ?
|
14, because:
KOH (aq) ----> K+ (aq) + OH- (aq) 1.0 M 1.0 M pOH = -log[OH-] = -log(1.0M) = -log(10^0 M) = 0 pH + pOH = 14 (at 25 degrees C) so pH = 14 - pOH =14 - 0 =14. |
|
|
What is pH for 1.0 M strong base?
|
14. Memorize this.
|
|
|
What is pH for 1.0 M strong acid?
|
0. Memorize this.
|
|
|
What is a weak acid/base?
|
acids and bases that are partially, reversibly dissociate (to show this a double arrow is put in weak acid or weak base reactions)
|
|
|
Given that K(aHF) = 7 x 10^-4, what is the pH of 1.5 M HF (aq)?
|
pH = 1.5
Because: HF (aq) <----> H+ (aq) + F- (aq) K(aHF) = [H+][F-] _______ [HF] (7 x 10^-4) = (x)(x) ________ (1.5 M - x) BECAUSE [HF] is more than 1000 times larger than K(aHF), we can ignore the fraction of HA lost due to dissociation... (7 x 10^-4) = (x)(x) ________ (1.5 M) x^2 = (7 x 10^-4) x (1.5 M) = 1 x 10^-3 x = 1 x 10^-1.5 so.. pH = -log[H+] = -log(1x10^-1.5 M) = 1.5 |
|
|
what is a conjugate pair?
|
two molecules with identical molecular formulas except that one of them has an additional H+.
|
|
|
HCL and CL-
H2) and OH- Na and NaOH.. are all examples of.. |
conjugate pairs
|
|
|
What are the conjugates of aqueous metal ions? (like Na (aq))
|
The conjugates are always the hydroxides...
so Na's conjugate would be Na(OH) think of Na (aq) as Na(H2O), so difference of one H+ is Na(OH) |
|
|
H3O/OH-
H2SO4/SO4 (2-) these are NOT examples of conjugate pairs because... |
differ by more than one H+
|
|
|
at 25 degrees Celsius, the sum of pK(a) and pK (b) of a conjugate pair for a weak acid & base must always equal _____
|
pH pK(a) + pK(b) = 14
|
|
|
What is ionic equilibrium?
|
because weak acids and bases do not completely ionize in aqueous solutions, it is the equilibrium between the weak acid and conjugate base, and the weak base and the conjugate acid.
|
|
|
Find equilibrium constants for weak acid and weak base NH4 + and NH3.
|
pK(aNH4+) + pK(bNH3) = 14
explanation: NH4+ = weak acid NH3 = weak base -NH4+ (aq) <---> H+ (aq) + NH3 (aq) -NH3 (aq) + H2O (l) <---> NH4+ (aq) + OH - (aq) K(aNH4+) = [NH3][H+]/[NH4+] K(bNH3) = [NH4+][OH-]/[NH3] Rearrange. [NH4+] = [NH3][H+]/K(aNH4+) [NH4+] = K(bNH3)[NH3]/[OH-] Set to equal each other [NH3][H+]/K(aNH4+) = K(bNH3)[NH3]/[OH-] Cancel out [NH3]'s. K(aNH4+) * K*(bNH3) =[OH-][H+] @ 25 deg. C [OH-][H+] = 14 therefore K(aNH4+) * K*(bNH3) = 14 |
|
|
What are the four conjugate rules?
|
1) the conjugate acid of a strong base is neutral (e.g. Na, the conjugate acid of NaOH is neutral).
2) The conjugate base of a strong acid is neutral. (e.g. Cl-, the conjugate base of HCl, is neutral) 3) The conjugate acid of a weak base is an acid. (e.g. NH4+, the conjugate acid of NH3, is acidic) 4) The conjugate base of a weak acid is a base. (e.g. F-, the conjugate base of HF, is basic) |
|
|
How do you calculate the pH of buffers?
|
pH = pK(a) + log [conjugate acid]
____________ [conjugate base] or pH = pK(a) + log [A-] _____ [HA] pH = pK(b) + log [conjugate base] ______________ [conjugate acid] pH = pK(b) + log [HA] ____ [A-] |
|
|
how does diluting a buffer affect its pH?
|
It doesn't change its pH whether you concentrate or dilute it!
|
|
|
Given the K(a-acetic acid) = 1.8 x 10^-5, what is the pH of a solution of 0.1 M acetic acid and 0.01 M sodium acetate?
|
pH = 3.7
ignore Na, because it is a conjugate acid of a strong base. HC2H3O2 (aq) <----> H+ (aq) + C2H3O2- (aq) therefore pH = pK(a-acetic acid) + log ([C2H3O2-]/[HC2H3O2]) pH = 4.7 + log ([0.01 M]/[0.1 M]) pH = 4.7 + log 10^-1 = 4.7 + (-1) =3.7 |
|
|
What do acids taste like?
|
taste sour
|
|
|
What do bases taste like?
|
taste bitter
|
|
|
what is a henderson-hasselbach equation used for?
|
to find pH of buffers.
|
|
|
What if you are given moles to use for a henderson -hasselbach equation?
|
pH = log (moles(A-)/moles(HA))
|
|
|
in henderson-hasselbach equation if the number of HA and A- moles are equal then what can the equation be simplified to?
|
pH = pK(a)
OR pOH = pK(b) |
|
|
What can acid - base titrations be used for?
|
1. find concentration of acid or base
2. whether an unknown acid or base is strong or weak 3. pK(a) of an unknown acid of pK(b) of an unknown base |
|
|
What is the equivalence point?
|
the point in titration where just enough titrant (in moles) has been added to completely neutralize the subject acid or base.
|
|
|
What is the end point?
|
synonymous with equivalence point
|
|
|
what is the inflection point?
|
synonymous with equivalence point
|
|
|
Is the equivalence point always neutral?
|
no, because although the unknown acid/base is neutralized, the conjugate acid or conjugate bases don't have to be neutral
|
|
|
What happens at the equivalence point in terms of conjugate acids & bases?
|
All the subject acid/base and titrant acid/base have been neutralized into conjugate acids/bases.
|
|
|
At equivalence point, how do you find unknown concentration of subject?
|
Molarity (subject)=
(molarity titrant * volume titrant) /Volume(subject) |
|
|
NaOH:
M = 0.1 v = 100 mL HNO3: v = 100 mL Find M |
M(HNO3)=(0.1 M * 0.1 L)/(0.1L)
=0.1 M. |
|
|
If the pH at equivalence point is exactly 7, what does this mean in terms of the unknown acid or base?
|
it is a strong acid or base
|
|
|
If the pH at equivalence point is greater or less than 7 the unknown acid or base is:
|
weak.
|
|
|
The equivalence point is at pH = 9, where the titrant was NaOH, therefore the subject is?
|
the original solution contained a weak acid!
|
|
|
What do you do to find the pK(a) or pK(b)using titration?
|
look at half equivalence point.
pH(at half point) = pK(a) OR pOH(at half point) = pK(b) |
|
|
What is used to add acid to base or base to acid?
|
a buret
|
|
|
An acid base indicator changes color in a +/-1 range of its
|
pK(a)
|
|
|
At pH = <7, litmus is ___
At pH = >7, litmus paper is __ |
pH = <7 = red
pH = >7 = blue |
|
|
An atom that loses electrons is ___
|
oxidized
remember LEO says GER |
|
|
An atom that gains electrons is ____
|
reduced
remember LEO says GER |
|
|
What are the two rules about oxidation states?
|
1)the atoms in any compound can be assigned oxidation states, and the charges reflect the atoms electronegativities.
2)For any compound, the total number of electrons given up is the same as the number gained. Thus oxidation states in neutral compound always add up to 0. |
|
|
What oxidation state are atoms in their elemental forms?
|
0
|
|
|
What are the 5 oxidation state rules you need to know for the chem SAT test?
|
Oxygen: -2 (unless peroxide like H2O2, at which it is -1)
Alkali metals: +1 Alkalie Earth metals: +2 Halogens: -1 Hydrogen: +/-1 |
|
|
What is the oxidation state for nitrogen in the compound nitrogen monoxide (NO)?
|
Oxygen is -2, so because must add to 0, N must be +2.
|
|
|
What is the oxidation number of carbon in iron(III) carbonate, Fe2(CO3)3?
|
+4.
First isolate CO3. Crossing subscripts we get CO3 as having a charge of 2-. So 1 C, and 3 O's must add up to a -2 charge. We know O's are -2 unless with a peroxide. Therefore the charge from O's are -6. In order to obtain a charge of -2, we must have +4. Therefore C is +4. |
|
|
Which are organic compounds more soluble in: non polar or polar solvents?
|
non polar
|
|
|
Do organic compounds dissociate in solution? what does this mean?
|
No, because they do not contain ionic bonds. This means they are poor conductors of electricity.
|
|
|
Two compounds have the same chemical make up with identical constituent elements, but are in a different geometrical arrangement, what are they called?
|
isomers.
And they can have totally different chemical properties. |
|
|
All living things on Earth are made up of this element:
|
carbon
|
|
|
Organic molecules almost always contain ___ bonds.
|
non polar covalent bonds
|
|
|
What is a hydrocarbon?
|
compounds that contain only carbon and hydrogen
|
|
|
What are the simplest organic compounds?
|
hydrocarbons.
|
|
|
What is an alkane?
|
hydrocarbons that contain only single bonds, also known as saturated hydrocarbons because each carbon is bonded to as many other atoms as possible.
|
|
|
What is are alkenes?
|
hydrocarbons that contain at least one double bond, also known as unsaturated.
|
|
|
What are alkynes?
|
hydrocarbons that contain triple bonds, can also be known as unsaturated (just like alkenes)
|
|
|
What is a hydrocarbon ring?
|
instead of a chain, carbons form a ring, such as benzene, C6H6.
|
|
|
What are the three types of hydrocarbons?
|
Alkanes, alkenes and alkynes.
|
|
|
What are the 8 main types of functional groups?
|
Alcohol, halides, organic/carboxylic acids, amines, aldehydes, ketones, ethers, and esters
|
|
|
What are characteristics of an alcohol?
|
- an O-H bondede to a carbon atom
-because of hydroxyl group, alcohols are POLAR -ends with "ol", like methanol |
|
|
What are characteristics of halides?
|
-halogen bonded to a carbon atom
-has halogen in name like "carbon tetraCHLORIDE" or with prefix "fluoro" etc. |
|
|
What are characteristics of organic/carboxylic acids?
|
-a carboxyl group bonded to a carbon chain
-H dissociates so these are ACIDIC -names are "___ic acid" |
|
|
What are characteristics of amines?
|
-an amine (NH2) bonded to a carbon atom
-amine group similiar to NH3 so BASIC -have "amine" in name |
|
|
What are characteristics of aldehydes?
|
-a carbonyl group (C=O) bonded to a carbon at the END of the carbon chain
-have "aldehyde" in name |
|
|
What are characteristics of Ketones?
|
-a carbonyl group (C=O) that is not at the end of the carbon chain
-have "one" at the end, like hexone |
|
|
What are characteristics of ethers?
|
-an oxygen atom links two hydrocarbon chains, think of biology, carbs bond through ether linkage
-have "ether" in name like dimethyl ether. |
|
|
What are characteristics of esters?
|
Same as ether except linked to a carbonyl (C=O) group
-has "ate" in name like methyl formate |
|
|
What is an addition organic reaction?
|
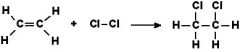
Occurs when a double bond is converted to a single bond, freeing space for other elemtns to bond. Can also be a triple bond being converted into a double bond.
|
|
|
What is a substitution organic reaction?
|
An atom or group in a compound is replaced with another atom or group
-very rare -Think of it as a single or double displacement reaction |
|
|
What is a polymerization reaction?
|
Two smaller compounds called monomers are joined to form a polymer
-think of biology with glucose and glucose (condensation polymerization) where an H2O is also produced |
|
|
What is a cracking reaction?
|
A large compound is broken down into a smaller compound
|
|
|
What are the main types of organic reactions?
|
Addition, substitution, polymerization, cracking, oxidation, esterification and fermentation
|
|
|
What is an oxidation reaction?
|
An organic compound can react with oxygen at high temperature to form carbon dioxide and water
-combustion or burning -e.g. CH4 +2O2 --> CO2 + 2H2O |
|
|
What is esterification?
|
-an organic acid reacts with an alcohol to produce an ester and water
e.g. CH3-O-OH + HOCH2CH3 ---> CH3-O-OCH2CH3 + H2O |
|
|
What is fermentation?
|
an organic compound reacts in the ABSENCE of oxygen to produce an alcohol and carbon dioxide.
|
|
|
What is a lipid?
|
-made up of carbon, hydrogen, and oxygen, in branching chains
-triglycerides are made up of three fatty acids and a glycerol -molecules that make up cell walls are phospholipids -they are not water soluble |
|
|
What are carbohydrates?
|
-polymers made up of sugar monomers (monosaccharides)
-storage of energy for animals is glycogen and cellulose for plants |
|
|
What are nucleic acids?
|
-contain carbon, hydrogen, oxygen, nitrogen, and phosphorus
-there are DNA and RNA |
|
|
what are proteins?
|
-polymers that are made up of amino acid monomers
-They have two functional groups, a corboxyl (-COOH) and amino group (-NH2), so they are amphoteric -All 20 amino acid monomers differ in their "side chain" or "R" group -chains of amino acids are known as polypeptides, and proteins are formed by folded polypeptides -enzymes which are biological catalysts, speed up cellular reactions and are all proteins |
|
|
What makes up the Earth's atmosphere?
|
-78% nitrogen
-20% oxygen -<1% argon -variable amounts of water variable |
|
|
What are the 4 components of the atmosphere?
|
-troposphere is closest to Earth
-stratosphere, above troposphere -mesosphere, above stratosphere -thermosphere which is furthest out |
|
|
What is ozone and how is it created?
|
O3.
results from collision of elemental oxygen and diatomic oxygen |
|
|
What is photodissociation?
|
bond is broken as a molecule absorbs a photon of light energy.
this is how diatomic oxygen is broken into elemental oxygen |
|
|
what are chlorofluorocarbons?
|
used in spray cans and car air conditioners
-react with light energy to form free chlorine, which reacts with the ozone, destroying it -primary destroyer of ozone |
|
|
What is the greenhouse effect?
|
absorption of infrared radiation from green house gases
|
|
|
Which chemicals tend to cause acid rain?
|
SO2 --> SO3 (g) + water becoming H2SO
OR nitrogen oxides like |
|
|
what's an example of acid rain?
|
SO3 (g) + H2O (l) --> H2SO4 (aq)
|
|
|
What is carbon monoxide?
|
comes from car exhaust/cigarette smoke
-binds irreversibly to hemoglobin (the biomolecule that distributes oxygen throughout body) |
|
|
name 5 safety rules.
|
-always wear safety goggles in lab
-always work with good ventilation -be diligent when working with a flame -when diluting acid, always add acid to the water to avoid splattering solution -when heating substances do it slowly to prevent spatter, or explosions |
|
|
what's the difference between accuracy and precision?
|
accuracy is how close a value is to accepted value
-precision is repeatability (if you do an experiment many times and get close answers to each other, but they don't have to necessarily be close to the accepted value) |
|
|
What are three methods of separation?
|
filtration, distillation and chromatography
|
|
|
Describe filtration
|
-solids are separated from liquids as mixture is passed through filter
-to find amount of solid that is filtered out, dry out filter (usually porous paper), and then weigh it subtracting weight of filter |
|
|
Describe distillation
|
two or more liquids of differing boiling points are separated by making temperature at boiling point of one liquid, but not for the other one, vaporizing just that one liquid
|
|
|
Describe chromatography
|
-substances are separated by differences in attraction to a surface
-substance more attracted move slower, separating two parts |
|
|
how does phenolphthalein work?
|
clear in neutral and acidic
-pink/magenta in basic solution |
|
|
How does litmus work?
|
pink in acidic and blue in basic solution
|
|
|
How do you use precipitation to identify chemicals?
|
-use process of elimination using solubility rules to identify chemicals
-e.g. chlorine is soluble with pretty much everything except silver chloride, so if a precipitate forms when chlorine is put in solution then it's likely silver |
|
|
How do you use conduction to identify chemicals?
|
to see if a solution contains ions or not you can check if the solution conducts electricity
-if it conducts its an ionic solute |
|
|
How do you use flame tests to identify chemicals?
|
salts of these elements burn...
lithium, strontium - red calcium-orange sodium - yellow barium - green potassium - violet |
|
|
How do you use colored solutions to identify chemicals?
|
color of a solution can be indicative of what elements are present
|
|
|
How do you use gas evolution to identify chemicals?
|
using a manometer one can find the amount of gas in a reaction.
-a erlenmeyer flask is hooked up to a U shaped tube filled partially with mercury, as the reaction proceeds the more gas, the higher the mercury rises up the tube |
|
|
How do you use calorimetry to identify chemicals?
|
used to find change in heat after reaction
-calorimeter used, where insulated very well, the thermometer measures change in temperature -taking change in T, mass and specific heat we can find heat energy |
|
|
Bases do what with unshared electron pair?
|
donate.
they accept protons, therefore donate electron pairs |
|
|
Acids do what with unshared electron pair?
|
accept.
They donate protons, so accept electron pairs |

