![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
32 Cards in this Set
- Front
- Back
|
lit is the backbone of compounds of organic and inorganic |
carbon |
|
|
contains carbon - carbon bonds |
organic chemistry |
|
|
types of chemical bonding |
covalent, ionic, metallic |
|
|
two non metal sharing |
covalent bonding |
|
|
equal sharing of electron |
non-polar covalent bond |
|
|
non-equal sharing of electron |
polar covalent bonding |
|
|
metal and non-metal transfering |
ionic bonding |
|
|
metal and metal pooling |
metallic bonding |
|
|
outermost electrons |
valence |
|
|
works when two stable charge or ions (negative + positive) |
conductivity |
|
|
like dissolves like |
solubility |
|

|
vital force |
|
|
how many cpds were discovered by the chemist and how many new ones divovered every year |
10 million, 10 thousand |
|
|
two principal ways to obtained organic compounds |
isolation from natured and synthesis in the laboratory |
|
|
types of isolation from nature |
plants, animals, natural gas, petroleum, oil shale distillate, coal |
|
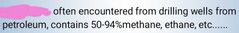
|
natural gas |
|

|
Taxus Brevifolia |
|
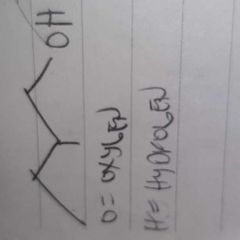
|
alcohol |
|

|
ether |
|
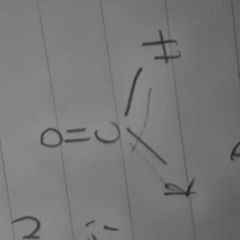
|
aldehyde |
|
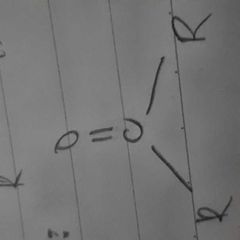
|
ketones |
|
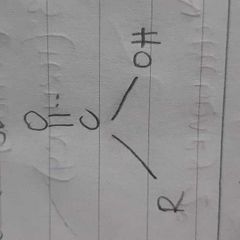
|
carboxylix acid |
|
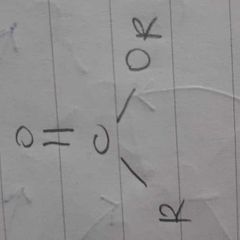
|
ester |
|
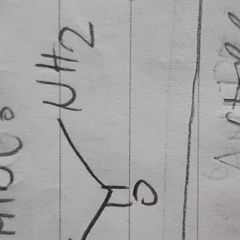
|
amide |
|
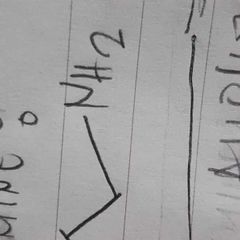
|
amine |
|
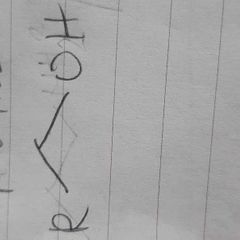
|
alcohol |
|

|
general formula |
|

|
molecular formula |
|

|
skeletal formula |
|

|
structural formula |
|

|
condensed structural formula |
|

|
line-angle formula |

