![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
73 Cards in this Set
- Front
- Back
|
Discuss Effective nuclear change?
|
-estimate zeff with Slaters rules |
|
|
Discuss Orbital energies?
|
-2s is more penetrating than 2p with the energy gap increasing with increasing atomic number |
|
|
Equation for orbital energy? |
E = - Zeff²RH/n² |
|
|
Discuss atomic radii?
|
-increase down a group, decrease across period -Ga<Al due to transitinon metal contraction |
|
|
Discuss Ionisation energy?
|
-Ga>Al -TM contraction -Be>B - 2p higher energy than 2s -N>O - exchange energy |
|
|
Discuss electronegativity?
|
-Ga> Al, Si> Ge TM contraction |
|
|
Discuss elements in top right corner?
|
small radii, High IE, Highe electron affinity, readily form anions |
|
|
Discuss elements in bottom left corner?
|
Large radii, low IE's readily form cations |
|
|
Discuss group 1 metals?
|
=> chemistry is dominated by tendency to lose a single s electron => less variation in chemistry than for elements of other main groups |
|
|
∆χ =0-0.5 |
covalent non-polar |
|
|
∆χ=0.5-1.8
|
covalent polar |
|
|
∆χ>2.0 |
Ionic |
|
|
Discuss group 1 halides?
|
for ∆Hf⁰: -MX becomes less negative F->Cl->Br->I -MX becomes more negative Li->Na->K->Rb->Cs except for X = F which is the opposite due to small size of F⁻ |
|
|
Born Haber Cycle (constituents)?
|
∆fH⁰ = ∆vapH⁰ + ∆ionH⁰ + ½∆dissH⁰ + ∆EAH₀ -∆lattH⁰ |
|
|
Group 1 oxides and hydroxides?
|
-Li gives oxides -Na gives oxides and peroxides -K gives peroxides and superoxides -Rb and Cs gives superoxides |
|
|
Group 1 hydrides?
|
-strong bases -ionic -NaH is a common strong base, nuceophile, reducing agent |
|
|
alkyl lithium?
|
-polarised bond -MeLi acts a nucleophile and base -tetrameric (MeLi)₄ -4 centre 2 electron bond at each Li₃ face |
|
|
How do we know if reaction is favourable from its ∆E?
|
∆G = -nFE⁰
|
|
|
Discuss group 2 alkaline earth metals?
|
-chemistry is dominated by formation of +2 ions -all form M²⁺ -small size of Be²⁺ means covalent compounds dominate for this compound (and to a lesser extent magnesium) |
|
|
polarising power?
|
|
|
|
Polarisability?
|
the ability of an atom to be polarised by a neigbouring atom (how easily electron density around an atom is distorted) |
|
|
Discuss group 2 halides?
|
-Chlorides, bromides and iodides of Mg, Ca, Sr and Ba are all ionic, water soluble salts -conduct in the melt -Fluorite structure is adopted by many MX² solids BeCl₂: -long polymer chain in solid state -monomer or dimer in gas phase -does not conduct electricity in the melt -soluble in donor organic solvents |
|
|
explain BeCl₂?
|
Be is Td ∴ sp³ Be-Cl-Be angle = 82 ∴ p orbitals (∼90) -one normal 2 centre - 2e⁻ bond -one dative 2 centre - 2e⁻ bond (lone pair on Cl) -The Cl is bridging -gives a polymeric structure -BeCl₂ is a lewis acid therefore lewis bases will disrupt dative Cl-> Be bond and hence can be dissolved in donor organic solvents |
|
|
Group 2 hydrides?
|
-BeH₂ and to a lesser extent MgH₂ are covalent -BeH₂ -linear gas phase H-Be-H, thermally stable polymeric structure in solid state |
|
|
Reason BeH₂? |
Be is Td ∴ Sp3 -Be-H-Be 3 centre 2e⁻ bonds -electron deficient ∴ reacts with lewis bases and is less leactive to water |
|
|
alkyl beryllium?
|
-CH₃ is isolobal with H -bulkier alkyls are dimeric or linear depending on steric congetstion -3c 2e⁻ bond |
|
|
Grignards?
|
RMgXs readily solvated by ether giving four coordinate Mg centres -at high concentrations can condense into polymeric structures formed by displace ment of a solvent molecule by the lone pair on a halide |
|
|
Group 2 carbonates?
|
MgCO₃, CaCO₃ and SrCO₃ are stable but decompose with heat BaCO₃ is stable to heat -down group, M less electronegative, less polarising, decomposition requirers higher temperature -Lattice enthaply is highest for small cation + small anion |
|
|
Solubility of salts in water?
|
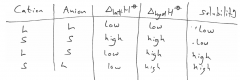
|
|
|
Discuss group 13?
|
-TM/lanthanide contraction explains deviations from expected trends in properties -all elements adopt +3 oxidation states, -+1 oxidation state becomes more prominent down the group (inert pair effect) |
|
|
The alternation effect?
|
(transitionn metal/ d block/ lanthanide contraction) e.g. Al < Ga (sum of first three ionisation energies) Ga has additional protons in nucleus, d electrons poorly shielded => strong attraction between nucleus and 4p¹ electron => increase in Ionisation energy Ln -> Tl same but for 4f¹⁴ electrons are poorly shielded |
|
|
inert pair effect?
|
e.g. Tl 6s orbitals low in energy and penetrating ionisation energy not compensated by lattice enthalpy of e.g. TlCl₃ ∴ +1 oxidation state preferred |
|
|
B₂H₆ structure and bonding?
|
-4 Terminal 2 centre 2 electron bonds -2 bridging 3 centre 2 electron bonds |
|
|
Group 13 halides?
|
-BX₃ displays significant π-bonding which is partly responsible for the planar structures due to its empty p orbital which can accept lone pairs |
|
|
AlF₃?
|
forms infinite salt structure with octahedral coordinated metal ions |
|
|
AlBr₃?
|
-exists in dimeric form with dative bonding from Br to empty Al orbitals similarly with AlI₃ |
|
|
AlCl₃?
|
ionic in the solid state but form dimers in the melt and in the gas phase ∆χ = 1.55 (border between ionic and covalent |
|
|
Frustrated Lewis Pairs?
|
trivalent phosphines are lewis bases -bulky groups attached to B and P hinder adduct formation giving a highly reactive species which can heteroyltically split H₂ giving a main group hydrogenation catalyst -used to activate alkynes and CO₂ |
|
|
Group 14?
|
oxidation states of +4 and +2 common, with +2 becoming more prevalent down the group (inert pair effect) +3 oxidation rare, found only in compounds with E-E or radical systems -Chemistry dominated by covalent compounds |
|
|
Group 14 bond hydrides?
|
-Boiling point of hydrides increases down the group due to increased van der Waals |
|
|
Group 14 halides and oxides?
|
-boiling point decreases down due to increased van der waals -CX₄ compounds are lower than expected due to steric demand around small C -Si often higher bond energies than expected due to d orbital interections |
|
|
Bonding in silicon compounds?
|
-SiO₂ favours polymer network of Si-O single bonds compared with CO₂ because Si=O<2Si-O while C=O > 2C-O |
|
|
Thermodynamic and kinetic stability of group 14?
|
-methane is thermodynamically unstable but it is kinetically inert -SiH₄ is spontaneously flammable in air - kinetically labile -same for halides in water -CCl₄ is stable in water -SiCl₄ reacts vigorously in water H₂O struggles to attack C due to size of Cl groups |
|
|
Carbenes?
|
-trigonal sp² hydridised -can be singlet or triplet dependant upon groups attached (CCl₂ - singlet ground state, R₂C - triplet gound state) singlet leaves empty orbital for π donation into it -Applications as ligands for transition metals in catalysis |
|
|
Carbides?
|
-saline carbides - group 1 and 2 (and Al) -metallic carbides - transition metals -metalloid carbides - boron and silicon |
|
|
Saline Carbides?
|
-Formed from graphite and metal vapour or metal dissolved in NH₃ -strong reducing agents Dicarbides (acetylides) -formed by reaction of metal and carbon at very high temperature -Ionic structure with anion C₂²⁻ Methides -Be₂C and Al₄C₃ -borderline between saline and metalloid -directional bonding implies not purely ionic |
|
|
Catentation?
|
E-E bond energies decrease down group -important for C and Si, less so for Ge and SN (C₆₀, C₇₀, buckytubes etc) (SiMe₂)n for n = 5-100 (GenH2n+2) for n = 1-10 SnnH2n+n for n = 1-6 |
|
|
Group 14 double bonds?
|
-Bulky R groups are required to favour double bonded molecules of the heavier group 14 elements |
|
|
Group 15?
|
-lower down group +3 oxidation is most stable due to inert pair effect |
|
|
allotropes?
|
different structural forms of the same element in which the chemical bonding is different |
|
|
Group 15?
|
N -> non-metal Bi-> characteristic properties of mani-group metal oxidation = -3 up to +5 Bi +3 most stable due to inert pair effect |
|
|
Allotropes of group 15?
|
P: loads and loads of them As, Sb, Bi: less than P |
|
|
White phosphorus?
|
-highly strained 60 bond angle -highly reactive (spontaneously ignites in air, burns under water) giving phosphorous acid (H₃PO₃)in low oxygen, and phosphoric acid (H₃PO₄) in excess oxygen |
|
|
Nitrogen oxides?
|
-variety of oxidation states, -π-bonding due to efficient overlap of 2p orbitals |
|
|
Group 15 halides?
|
-pentavalent halides for P, As, Sb, Bi not N due to size -have to use MO theory to explain many properties as hybridisation is not very useful |
|
|
Hypervalency?
|
possible for large elements due to size and access to D orbitals
|
|
|
Boron-Nitrogen compounds?
|
-Boron Nitride B-N-B-N-B -graphite like compound -diamond like compound -borazane aka amino boranes |
|
|
Nitrogen - Phosphorus compounds?
|
phosphazenes |
|
|
Group 16?
|
-high electronegativity of O means +2 is max oxidation state |
|
|
oxygen allotopes?
|
O₂ and ozone |
|
|
Sulfur?
|
-all forms contain S-S single bonds like S₈ rings |
|
|
Group 16 hydrides?
|
-E-H bond enthalpy decreases down group - poor orbital overlap -H-E-H bond angle decreases due to less s-p mixing |
|
|
Group 16 halides?
|
-sulfur fluorides unstable towards hydrolysis with exception of SF₆ |
|
|
Group 16 polyanions and polycations? |
S₈²⁺ Se₄²⁺ cyclic /bicyclic can be prepared |
|
|
SxNx compounds?
|
-extremely sensitive to explosive decomposition -orange solid -cradle shaped with weak bonding interactions between S atoms across the ring poly(sulfur nitride) -one-dimensional polymer -coducting at room temperature, super-conductor at liquid He temperatures -inert to hydrolysis, -slowly decomposed by hydroxide |
|
|
Group 17?
|
-F always -1 and able to stabalize high oxidation states of other elements -poorer overlap for increasing size of atoms (with more diffuse orbitals) down group, decreasing bond enthalpy, exception F which has a really low bond enthalpy |
|
|
Group 17 hydrides?
|
-HF is a volatile liquid wheras other HX are gases at RT -HF is extremely toxic and corrosive, able to attack glass, metals, concrete and bone |
|
|
Group 17 interhalogens?
|
XY, XY₃, XY₅ and XY₇ |
|
|
polyiodides?
|
-sensitive to the countanion -bond length longer than in I₂ |
|
|
Hypervalent iodine compounds?
|
alternative to metal -based oxidising agents -lower toxicity -easier to handle -milder reaction conditions |
|
|
Group 18?
|
Xenon has developed covalent chemistry |
|
|
Group 18 elements?
|
-He used as coolant -Ne, Kr used in lighting -Ar used as inert gas -Xe propulsion of ion engines -Rn radioactive so not investigated |
|
|
Xenon?
|
-XeF₂, XeF₄, XeF₆ -all reactive, highly oxidising xenon oxides -XeO₃ -explosize and highly oxidising |

