![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
Element |

Pure substance that cannot be changed into a simpler substance, all one type of atom. |
|
|
Compound |

Pure substances made up of one or two types of elements that are chemically combined. Can only be changed into simpler substances through chemical changes. |
|
|
Mixture |

Physical combinations of two or more types of substances that retain their own properties and are mixed together; they can only be separated through physical means. |
|
|
Atom |
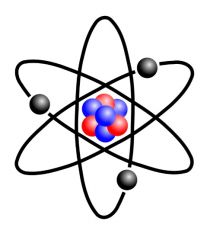
Building block of matter and made up o particles too small to be seen with an optical microscope. |
|
|
Physical Property |
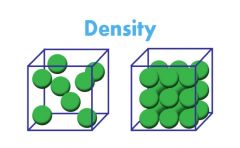
A property that can be observed without changing the substance into a new substance. Ex: density, texture, color, magnetism, boiling point |
|
|
Chemical Property |

A property that can only be observed by changing the substance. Ex: flammability, oxidation. |
|
|
Solid |

A form of matter where the atoms and molecules are tightly packed and can only vibrate. |
|
|
Liquid |

A form of matter where the atoms and molecules can collide with ad move past each other. |
|
|
Gas |

A form of matter where the atoms and molecules move independently, colliding frequently. |

