![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
84 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
what's an example of a chemical change
|
adding baking soda to vinegar in an attempt to make a small erupting "volcano" of foam
|
|
|
|
a student obtains 2.50g of aspirin in lab. he calculated ahead of time that he should make 2.65g. what is his theoretical yield?
|
2.65gpro
|
|
|
|
property of sulfur
|
brittle
|
|
|
|
heterogeneous mixture
|
sAlsa
|
|
|
|
example of potential energy
|
water at the top of a waterfall
|
|
|
|
example of a physical change
|
shredding paper
|
|
|
|
example of a homogeneous mixture
|
steel
|
|
|
|
classified as an element
|
helium
|
|
|
|
true statement
|
your weight is the result of the pull of gravity & does not change your mass
|
|
|
|
energy
|
the capacity oR ability to do work
|
|
|
|
Daytons atomic theory originally stated in 1808
|
atoms can be subdivided, created, & destroyed
|
|
|
|
theoretical yield
|
the amount of product a student was supposed to produce, as calculated ahead of time
|
|
|
|
gamma ray
|
form of energy that can be emitted during radioactive decay that is a stream of electrons with very high energy
|
|
|
|
law of definite proportions
|
The elemental composition of a pure source is always the same, regardless of the source
|
|
|
|
law of multiple proportions
|
when two elements combine, they do so in ratios of small whole numbers and have a fixed weight
|
|
|
|
law of conservation of mass
|
matter can not be created nor destroyed
|
|
|
|
exothermic reactiob
|
in lab, sodium hydroxide was mixed with water. The temperature increased sharply.
|
|
|
|
endothermic reaction
|
in lab, ammonium nitrate was next with water. The temperature decreased sharply.
|
|
|
|
thomson
|
discovered the presence of the electron using a cathode ray tube and determined it's charge
|
|
|
|
what is the correct assumption regarding the gold-foil experiment?
|
The nucleus is very small compared to the energy levels and positively charged
|
|
|
|
true statement
|
protons and neutrons have approximately the same mass while the electron is lighter
|
|
|
|
isotope
|
have the same number of protons and electrons but different number of neutrons and have the same atomic number but different mass number
|
|
|
|
what has the mass number of 40?
|
calcium
|
|
|
|
how many neutrons does uranium – 238 have?
|
146
|
238-92=146
238-(atomic #)=146 |
|
|
what is the identity of the element represented by [Ne]3S23P5?
|
chlorine
|
|
|
|
electromagnetic spectrum
|
when wave lengths are short energy is high and when frequency is high energy is high
|
|
|
|
alpha particle
|
form of energy that can be emitted during radioactive decay that has the same mass number and atomic number of helium
|
|
|
|
Bohr's model of the hydrogen atom
|
electrons travel around the nucleus as the planets orbit the sun
electrons travel in fixed paths or orbitals a certain distance from the nucleus if an electron changes energy levels there is a quantum energy change when electrons drop from a higher energy level to a lower energy level they emit it energy from the form of a photon |
|
|
|
beta particle
|
form of energy that can be emitted during radioactive decay that has no Mass number but carries a -1 charge
|
|
|
|
and Atom with 16 protons and 17 neutrons would be an isotope of what element
|
sulfur
|
|
|
|
gamma rays
|
form of energy released during radioactive decay that can only be stocked by lead or very thick concentrate
|
|
|
|
halogens
|
Group of elements that is most active nonmetals all having seven valance electrons
|
|
|
|
noble gases
|
considered electronically perfect
|
|
|
|
The maximum number of electrons that the D sublevel can hold is
|
10
|
|
|
|
how many electrons, maximum, can the third main energy level hold
|
18
|
|
|
|
which one of the elements can be found in the P block
|
selenium
|
|
|
|
A member of the halogens
|
bromine
|
|
|
|
how many valence electrons does sulfur have
|
6
|
|
|
|
what is an example of a noble gas
|
argon
|
|
|
|
how many electrons remain unpaired for the element nitrogen
|
3
|
|
|
|
what is an example of a D block element
|
titanium
|
|
|
|
what quantum number will tell you the size of an Atom
|
principal
|
|
|
|
what call some number represents the direction in which the electrons are traveling and can also represent the electrons with arrows or +1/2, -1/2
|
spin
|
|
|
|
what is an example of the transition elements
|
copper
|
|
|
|
what's an example of the alkali metals
|
sodium
|
|
|
|
hunds rule
|
electrons remain unpaired as long as possible before pairing due to their like charges
|
|
|
|
Heisenberg principal
|
it's impossible to determine both the speed and location of an electron at the same time
|
|
|
|
Henry Mosley's work
|
elements show periodic repetition of properties
|
|
|
|
DeBrogile's work
|
electrons have wavelike behaviors and have certain frequencies
|
|
|
|
how many energy levels would you need to draw to accurately represent the element tin
|
5
|
|
|
|
what's an example of an alkaline earth metal
|
calcium
|
|
|
|
what's correct about most elements
|
large radius=low electronegativity= low ionization energy
|
|
|
|
how many valence electrons does calcium have
|
2
|
|
|
|
alkali metalw
|
Group of elements will form very strong bases and explode when placed in water
|
|
|
|
what is most likely to form an ionic bond
|
calcium and nitrogen
|
|
|
|
max planck
|
Hot objects when subjected to energy such as sunlight, emit it that energy in specific amount called quanta
|
|
|
|
Erwin schrodinger
|
stated that electrons have both wavelike properties and particle properties and developed an equation that treated electrons in atoms as waves
|
|
|
|
einstein
|
stated that electromagnetic Bridget has wavelike properties, that particle stream, and that each particle of like carried a quantum of energy
|
|
|
|
bohr
|
electrons travel a specific distance from the nucleus like planets orbiting the sun
|
|
|
|
what's most likely to form a covalent bond
|
carbon and chlorine
|
|
|
|
what will not form a cation
|
fluorine
|
|
|
|
what property in general increases as you move left to right across the periodic table in a period?
|
electronegativity and ionization energy
|
|
|
|
what intermolecular force produces an instantaneous dipole do you to call sinc swirl of electrons his forces get stronger as the molecules increases and weight?
|
London dispersion
|
|
|
|
salt bridge
|
A piece of paper soaked in salt water that allows ions to flow BU does not allow to solutions to mix
|
|
|
|
what intermolecular forces found between polar molecules only and has a short range and produces compounds with very high boiling points
|
dipole – dipole all
|
|
|
|
what type of intermolecular force is found between hydrogen and a very electronegative element
|
hydrogen
|
|
|

|
trigonal pyramidal
polar and soluble |
|
|

|
tetrahedral
nonpolar and nonsoluble |
|
|
|
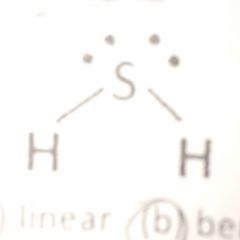
bent
polar and soluble |
|
|
|
what's the correct name for Cu(NO2)2
|
cupric nitrite
|
|
|
|
what's the correct name for the compound Cu2O
|
copper I oxide
|
|
|
|
what's the name of the compound C3O4
|
tricarbon tetroxide
|
|
|
|
what's the correct formula for carbon disulfide
|
CS2
|
|
|
|
what is calcium phosphate
|
Ca3
|
|
|
|
what's the name for Co2(SO4)3
|
cobalt III sulfate
|
|
|
|
what bond will there be a sharing of electrons producing an uneven distribution of charge in a molecule that is symmetrical
|
nonpolar covalent
|
|
|
|
what's the correct chemical formula for phosphoric acid
|
H3PO4
|
|
|
|
Who was the first scientist to publish the periodic table
|
mendeleev
|
|
|
|
in the double replacement reaction between silver nitrate and sodium iodide in lab, what is the spectator ions
|
sodium and nitrate
|
|
|
|
aq
|
dissolved in water
|
|
|
|
ppt
|
precipitate (substance produced is a solid)
|
|
|
|
---^-->
|
requires heat
|
|
|
|
---pt--->
|
reaction requires a catalyst
|
|
|
|
g
|
gas is produced
|
|

