![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
81 Cards in this Set
- Front
- Back
|
What's a hypothesis?
|
An educated guess.
|
|
|
What's scientific theory?
|
An explanation of why and how a specific natural phenomenon occurs.
|
|
|
What's Avogadro's Number?
|
6.02 x 10^23
|
|
|
What's double replacement?
|
A chemical reaction where two compounds are mixed and they exchange parts of their compounds with each other.
|
|
|
What does diatomic mean?
|
It's used to describe a molecule with more than one atom.
|
|
|
What's an empirical formula?
|
A chemical formula that shows the composition of a compound in terms of the relative numbers and kinds of atoms in the simplest ratio.
|
|
|
What's scientific law?
|
A logical, mathematical statement describing a consistency that applies to all members of a broad class of things.
|
|
|
What's the independent variable?
|
The variable that doesn't change.
|
|
|
What form of measurement is kelvin?
|
Degree, temperature.
|
|
|
What's an element?
|
A substance that cannot be broken down or separated by chemical means. All atoms of an element have the same atomic number.
|
|
|
What's an atomic mass unit or AMU?
|
It's a unit of mass used to express atomic and molecular masses.
|
|
|
What did the Aufbau Principle state?
|
Eelectrons fill orbitals starting at the lowest available (possible) energy states before filling higher states. Ex: 1s before 2s.
|
|
|
What did the Law of Octaves state?
|
The eighth element is a kind of repetition of the first,like the eighth note of an octave in music this is called Law of Octaves.
|
|
|
What does monoatomic mean?
|
A molecule has only one atom.
|
|
|
What are non-polar covalent bonds?
|
A covalent bond in which the bonding electrons are shared equally by the bonded atoms, resulting in a balanced distribution of electrical charge.
|
|
|
What is electron affinity?
|
The energy needed to remove an electron from a negative ion to form a neutral atom or molecule.
|
|
|
What are Lewis Dot Structures?
|
A structural formula in which electrons are represented by dots; dot pairs or dashes between two atomic symbols representing pairs.
|
|
|
What's amplitude?
|
Amplitude is the height difference to the peak and the trough of the wave.
|
|
|
What's chemical change?
|
A change that results in the formation of a new substance, such as burns or rust.
|
|
|
What's a physical change?
|
A change that doesn't effect the chemical composition of an object.
|
|
|
What is a mole?
|
A SI base unit used to measure the amount of a substance whose number of particles is the same as the number of atoms of carbon in exactly 12g of carbon-12.
|
|
|
What's single replacement?
|
One element replaces another in a compound.
|
|
|
What's a decomposition reaction?
|
A reaction in which a single compound breaks down to form two or more simpler substances.
|
|
|
What's an ionic compound?
|
A compound composed of ions bound together by electrostatic attraction.
|
|
|
What's molar mass?
|
The mas in grams of 1 mol of a substance.
|
|
|
What's an observation?
|
Something one notices.
|
|
|
What's the dependent variable?
|
The variable that is changed for experimental purposes.
|
|
|
What is Celsius?
|
A measurement of temperature, the base unit used in chemistry.
|
|
|
What's a heterogeneous mixture?
|
A mixture composed of dissimilar components.
|
|
|
What's a homogeneous mixture?
|
A mixture that has uniform structure throughout.
|
|
|
What's a Quanta?
|
.
|
|
|
What does the Pauli Exclusion Principle state?
|
That states that two particles of a certain class cannot be in exactly the same energy set.
|
|
|
What does malleable mean?
|
That a substance has the ability to be hammered or beaten into a thin sheet.
|
|
|
What is electronegativity?
|
A measure of the ability of an atom in a chemical compound to attract electrons.
|
|
|
What's a percent error?
|
The relative error.
|
|
|
What is electron configuration?
|
The arrangement of electrons in an atom.
|
|
|
What does Heisenburg's Uncertainty Principle state?
|
That the exact momentum and exact location of a particle cannot be specified.
|
|
|
What's precision?
|
Reproductability
|
|
|
What is accuracy?
|
The correctness of a single measurement.
|
|
|
What is a physical property?
|
A characteristic of a substance that does not involve a chemical change, such as density, color, or hardness.
|
|
|
What's a coefficient?
|
A small whole number that appears as a factor in front of a formula in a chemical equation.
|
|
|
What's a product?
|
A substance that forms in a chemical reaction.
|
|
|
What is Synthesis?
|
The formation of a complex product from simpler reactants.
|
|
|
What are molecular compounds?
|
Chemical compounds whose simplest units are in molecules.
|
|
|
What is molecular formula?
|
A chemical formula that shows the number and kinds of atoms in a molecule, but not the arrangement of the atoms.
|
|
|
What is absolute zero?
|
The temperature at which all molecular motion stops. (0 on Kelvin scale or -273.16C on Celsius scale)
|
|
|
What's a compound?
|
A substance made up of atoms of two or more different elements joined by chemical bounds.
|
|
|
What does Hund's Rule state?
|
That for an atom in the ground state, the number of unpaired electrons is the maximum possible and these unpaired electrons have the same spin.
|
|
|
What does Periodic Law state?
|
The physical and chemical properties of the elements are periodic functions of their atomic numbers.
|
|
|
What does it mean if a substance is ductile?
|
It means that it can be hammered thin or drawn out into a wire.
|
|
|
What is a polar covalent bond?
|
A covalent bond in which a pair of electrons shared by two atoms is helf more closely by one atom.
|
|
|
What is ionization energy?
|
The energy required to remove an electron from an atom or ion.
|
|
|
What is the electromagnetic spectrum?
|
All of the frequencies/wavelengths of electromagnetic radiation.
|
|
|
What is wavelength?
|
The distance inbetween two waves.
|
|
|
What's a photon?
|
A unit or quantum of light; a particle of electromagnetic radiation that has zero rest mass and carries a quantum of energy.
|
|
|
What is frequency?
|
The number of waves produced in a given amount of time.
|
|
|
What's a chemical property?
|
A property of matter that describes a substance's ability to participate in chemical reactions.
|
|
|
What is the formula for finding density?
|
D = m/v
Where D is density, m is mass, and v is volume. |
|
|
How many meters are in a dekameter?
|
10^1
|
|
|
How many meters are in a decimeter?
|
10^-1
|
|
|
How many meters are in a hectometer?
|
10^2
|
|
|
How many meters are in a centimeter?
|
10^-2
|
|
|
How many meters are in a kilometer?
|
10^3
|
|
|
How many meters are in a millimeter?
|
10^-3
|
|
|
How many meters are in a megameter?
|
10^6
|
|
|
How many meters are in a micrometer?
|
10^-6
|
|
|
How many meters are in a gigameter?
|
10^9
|
|
|
How many meters are in a nanometer?
|
10^-9
|
|
|
How many meters are in a terameter?
|
10^12
|
|
|
How many meters are in a picometer?
|
10^-12
|
|
|
How many meters are in a femtometer?
|
10^-15
|
|
|
How many meters are in an attometer?
|
10^-18
|
|
|
Know this.
|
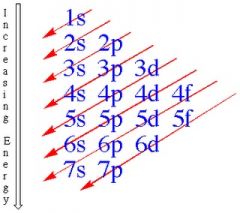
|
|
|
How does one find formula mass?
|
One would add all the molar masses together.
EX: K - 39.19 amu Cl - 35.45 amu O3 - 48.00 amu --------------- KClO3 - 122.55 amu |
|
|
Amount in Moles x Molar Mass (g/mol) = _________.
|
Mass in Grams
|
|
|
Mass in Grams x 1 / molar mass (g/mol) = __________.
|
Amount in Moles
|
|
|
Mass of Element in Sample of Compound
________________________ Mass of Sample of Compound x 100 = ____________. |
% Element in Compound
|
|
|
How does one determine a compound's empirical formula?
|
From its percentage composition, begin by converting that to a mass composition. Then, one would take the mass composition of each element and convert it to a composition in moles by dividing by the appropriate molar mass. Then divide by the smallest number between the two mole ratios. Then one can put it together from there.
|
|
|
What are the rules for assigning oxidation numbers?
|
-A free element's oxidation number will always be 0.
-Pay attention to the charge. Na+ becomes +1. -H is always +1. -O is always -2. -Group one (Alkali) metals are always +1. Group two (Alkaline-earth) metals are always +2. -Halogens are always -1. -The charge is the SUM. |
|
|
What does the extension OH mean?
|
Hydroxide.
|
|
|
How does one find the molecular formula of a compound?
|
One would first determine the mass of the empirical formula, then divide the molar mass by that, then multiply the result by the subscripts in the empirical formula.
|

