![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
Bronsted-Lowry acid definition
|
proton donor
|
|
|
Bronsted-Lowry base definition
|
proton acceptor, must have a lone pair of electrons
|
|
|
Bronsted-Lowry acid-base reaction definition
|
proton transfer process
|
|
|
Arrhenius acid definition
|
a substance with H in its formula that dissociates to yield H3O+
|
|
|
Arrhenius base definition
|
a substance with OH in its formula that dissociates to yield OH-
|
|
|
acid dissociation constant
|
Ka=[H3O+][A-]/[HA]
for the reaction HA + H2O -> H3O+ + A- |
|
|
Strong Acids
|
HCl, HBr, HI, HNO3, H2SO4, HClO4 (oxygen atoms exceed the number of ionizable protons by 2 or more)
|
|
|
Ion-product constant for water
|
Kw= [H3O][OH-] = 1.0x10^-14 at 25`C
|
|
|
pH
|
pH = -log [H3O+]
|
|
|
pKa
|
pKa = -logKa
|
|
|
pH, pOH, pKw relationship
|
pKw = pH + pOH = 14.00 at 25`C
|
|
|
mol/L*s
|
0 order
|
|
|
1/s
|
first order
|
|
|
L/mol*s
|
second order
|
|
|
L^2/mol^2 * s
|
3rd order
|
|
|
rate law for first order
|
ln [A] = kt + [A]
|
|
|
integrated rate laws
|
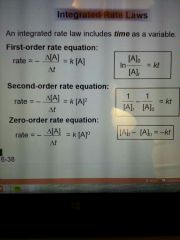
|

