![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
37 Cards in this Set
- Front
- Back
|
Definition: Relative molecular mass (mr) |
The relative molecular (or relative formula) mass is the average mass of a molecule of formula unit compared to 1/12th the mass of a carbon 12 atom |
|
|
Ar from Isotopic Abundances |
Multiply each relative isotopic mass by its %. Add up results then divide by 100 |
|
|
Relative atomic mass from a mass spectra graph |
Multiply each relative isotopic mass by it's relative isotopic abundance. Add up the results then divide by the SUM of the isotopic abundances |
|
|
Relative isotopic mass |
Mass of an atom of an isotope compared to the mass of 1/12th of a carbon 12 atom |
|
|
Generic moles equation |
N (moles) = m (mass) / Mr (relative atomic mass) |
|
|
Molar mass |
The mass of one mole in grams, the same as relative molecular mass |
|
|
Molar mass |
The mass of one mole in grams, the same as relative molecular mass |
|
|
Avogadro's constant |
Number of particles in one mole = 6.02 * 10^23 |
|
|
Equation for the number of moles from number of atoms/molecules |
Number of moles = number of particles you have / number of particles on a mole (av constant) |
|
|
Volume of gasses |
All gasses take up the same vol under the same conditions; 24dm^3 mol ^-1 Known as molar gas volume |
|
|
Formula for number of moles in a volume of gas |
Number of moles = volume in dm3 / Molar gas volume (24 dm3mol-1)
N.mol = v(dm3)/24dm3 mol-1 |
|
|
Formula for number of moles in a volume of gas |
Number of moles = volume in dm3 / Molar gas volume (24 dm3mol-1) |
|
|
Ideal gas equation |
pV = nRT
(P = pressure in Pa, V = volume in m3, n = number of moles, R = gas constant (8.314JK-1mol-1,) T = temperature in K (kelvin)) |
|
|
What is an ideal gas |
A gas where all particles have equal energy and none is lost or transferred (in super simple terms) |
|
|
What is the empirical formula |
Gives the smallest whole number ratio of atoms of each element in a compound. Smallest possible. |
|
|
What is the molecular formula? |
Gives the actual number of each type of atom in each molecule. Made up of a whole number of empirical units. Do not cancel down. |
|
|
Empirical formulae from experiments |

Back (Definition) |
|
|
Empirical formulae from experiments |

Use n=m/Mr to find moles then find a ratio. |
|
|
Empirical formulae from % |

Back (Definition) |
|
|
Molecular formula from experiential data |
**** u
What the **** is this?^^^ |
|
|
Balancing equations |

Balancing equations |
|
|
Ionic equatiosn |
Only show reacting particles and products formed Eg you might get: HNO3 + NaOH -> NaNO3 + H2O But you'd only write: H^+ + OH- = H2O |
|
|
Working out masses from balanced equations (calculate the mass of iron oxide produced when 28g of iron is burnt in air) |
2Fe + 3/2O2 -> Fe2O3 Mr of Fe = 55.8gmol^-1, moles of Fe = n=m/Mr, 28/55.8 = 0.5moles From equation - 2 mole of Fe= 1 mole of Fe2O3, so 0.50 moles = 0.25 moles of Fe2O3. Mr of Fe2O3 = (2*55.8)+)3*16)=159.6gmol-1 Mass of Fe2O3 = n*Mr = 0.25*159.6 = 40g |
|
|
Using balanced equations to work out gas volume (how much gas is produced when 15g of Sodium reacts with water at r.t.p? |
2Na + 2H2O -> 2NaOH + H2 Mr of Na = 23gmol-1, n of Na = 15/23.9 = 0.65moles From equation 2 moles of Na produce 1 mole of H2 0.65 moles of Na = 0.325 moles of H2 H2 = 0.325*24 (gas constant) = 7.8 dm3 |
|
|
Common ions |
Na -> e- + Na+, Mg -> 2e- + Mg2+, Cl + e- -> Cl-, O + 2e- -> O2-, |
|
|
Common ions |
Na -> e- + Na+, Mg -> 2e- + Mg2+, Cl + e- -> Cl-, O + 2e- -> O2-,
Tbh just look at the group they're in |
|
|
Some molecular (more than one element) ions to learn |
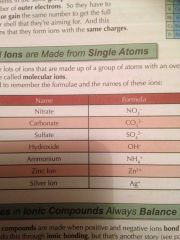
Back (Definition) |
|
|
Charges in ionic compounds always... |
Balance each other out. In NaCl the +1 charge in Na balances kHz the -1 charge in Cl, in MgCl2 the +2 Mg charge cancels out the two -1 charges in Cl. |
|
|
Salts as ionic compounds |
Acid + base -> water + salt Salts = lattice of positive and negative, sometimes (when hydrated) w/ water if crystallisation (w/out its called anhydrous.) |
|
|
Salts as ionic compounds |
Acid + base -> water + salt Salts = lattice of positive and negative, sometimes (when hydrated) w/ water if crystallisation (w/out its called anhydrous.) |
|
|
Moles and water of crystallisation |
One mole of a particular hydrated salt has the same number of moles of water of crystallisation. It's formula shows how many. |
|
|
Salts as ionic compounds |
Acid + base -> water + salt Salts = lattice of positive and negative, sometimes (when hydrated) w/ water if crystallisation (w/out its called anhydrous.) |
|
|
Moles and water of crystallisation |
One mole of a particular hydrated salt has the same number of moles of water of crystallisation. It's formula shows how many. |
|
|
Acids and bases |
- hydrated protons. |
|
|
Salts as ionic compounds |
Acid + base -> water + salt Salts = lattice of positive and negative, sometimes (when hydrated) w/ water if crystallisation (w/out its called anhydrous.) |
|
|
Moles and water of crystallisation |
One mole of a particular hydrated salt has the same number of moles of water of crystallisation. It's formula shows how many. Eg hydrated copper sulphate has 5 moles of water for every mole of salt, CuSO4.5H2O |
|
|
Acids and bases |
- acids are proton donors; when mixed w water they release hydrogen (H+) ions which are basically just protons - bases do the opposite, they take protons. Soluble bases (alkalis) release OH- atoms in water. |

