![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
How many molecules are in a mole? |
6.02 × 10^23 |
|
|
|
Equation for a mole |
n=mass÷Mr |
|
|
|
Equation for amount of particles |
Number of particles= n × avogadro's constant, 6.02×10^23 |
|
|
|
both equations for moles |
Volume × concentration= mass/Mr=n |
Amounts in solution |
|
|
What are the principle energy levels sub levels and how many electrons can they hold |
S, P, D, F , G/ 2,6,10,14,18 |
Up to 5 |
|
|
How do electrons fill sub levels first 5 |
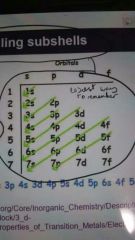
|
|
|
|
What can you use in replacement of the intial sub levels instead of writing them out |
The last noble gas in the periodic table before the element |
|
|
|
How to calculate a new concentration |
(Initial concentration x initial volume)÷final volume |
|
|
|
What are the quantam numbers |
n, principal/L Angular/ ml magnetic/ ms spin |
|
|
|
How would you record the ionsation energy for an element |
DeltaH=Delta,i (for reaction) 1 (for energy level) |
|
|
|
How does ionization energy change on the periodic table |
From bottom left to top right |
|
|
|
How does electron affinity change on periodic table |
Bottom left, to top right |
|
|
|
How does electronegativity change on a periodic table |
Increases from bottom left to top right |
|
|
|
How does the atomic radius change on the periodic table |
Smallest on the top right to largest on the bottom left |
|
|
|
What is enthalpy |
Energy exchange at a constant pressure |
|
|
|
On which reaction is delta H positive and which on is delta H negative |
Endothermic delta H positive, exothermic delta H negative |
|
|
|
Equations for enthalpy |
Sum of delta H bonds broken- sum of delta H bonds formed |
|
|
|
Name the enthalpy definitions and how you calculate them |

|
|
|
|
What does an energy cycle help you find |
Enthalpy in a reaction |
|
|
|
What is the general equation for heat change/ delta H |
Delta H = -q/n |
|
|
|
What is entropy |
A measure of the number of ways that particles can be arrange and the energy distibuted between them |
|
|
|
What is entropy at absolute freezing point |
0 |
|
|
|
Formula for entropy change |
Delta entropy= sum of products entropy- sum of reactants entropy |
|

