![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
28 Cards in this Set
- Front
- Back
|
What is the centre of an atom called? |
The Nucleus |
|
|
What is the charge of an electron? |
One Negative
Electrons are negatively charged sub-atomic particles. |
|
|
Which of the three sub-atomic Particles is the lightest? |
The Electron
The electron is so light compared to the proton and neutron that it has a negligible mass. |
|
|
If atoms contain charged particles, why do they not have a charge? |
They contain the same number of protons as electrons.
Atoms are neutral because they have equal numbers of protons (positive charges) as electrons (negative charges). |
|
|
What is the atomic number of an atom equal to? |
The number of protons in the nucleus.
The atomic number is equal to the number of protons in the nucleus of an atom. |
|
|
Where are the electrons inside an atom? |
They are arranged in energy levels.
Inside an atom, the electrons orbit around the nucleus and are arranged into energy levels. |
|
|
Which elements have similar chemical properties? |
Elements with the same number of electrons in their outer energy level.
Groups in the Periodic Table have elements with the same number of outer electrons and have similar chemical properties. |
|

How many neutrons does this atom of carbon have? |
8
The number of neutrons is calculated by subtracting the atomic number from the mass number. |
|
|
What are isotopes? |
Atoms with the same number of protons but a different number of neutrons.
Isotopes are atoms of the same element with the same atomic number but a different mass number. |
|
|
Hydrogen has three isotopes and a relative atomic mass of 1.0079. Which isotope is the most abundant? |
Hydrogen with atomic mass of 1.
The relative atomic mass takes into account the proportion of each element. |
|
|
Which of the following pairs are both diatomic elements? *Hydrogen and helium *Iodine and nitrogen *Boron and fluorine |
Iodine and nitrogen
The seven diatome elements are iodine, bromine, chlorine, fluorine, oxygen, nitrogen and hydrogen. |
|

How can the bonding in silicon dioxide (SiO2) be described? |
Covalent network
Silicon dioxide exists as a large 3D network of silicon and oxygen atoms joined together by covalent bonds. |
|
|
Why do ionic compounds not conduct when they are solid? |
Their ions are not free to move.
Ionic lattices will only conduct when the oppositely charged ions are free to move (ie molten or in solution). |
|
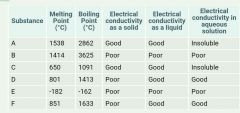
The table shows the properties of some different substances. Which substances are covalent? |
B + E
Both covalent molecular substances and covalent networks are poor conductors whether solid, molten or in solution. |
|

The table above shows the properties of some different substances. Which substances are ionic? |
D + F
Ionic substances have high MP and BP and are poor conductors when solid but conduct well when molten or in solution. |
|
|
In the compound tin(IV) chloride, a small group of atoms are held together by shared pairs of electrons. What type of bonding is present in tin (IV) chloride? |
Discrete covalent molecular
Since the atoms share electrons, the bonding is covalent. A small group of atoms can also be called a discrete molecule. |
|
|
Which of these compounds are made up of diatomic molecules? *Nitrogen monoxide (NO) *Magnesium oxide (MgO) *Carbon dioxide (CO2) |
Nitrogen monoxide (NO)
Nitrogen monoxide molecules are made from only two atoms - one of oxygen and one of nitrogen. |
|
|
Which of these does not have a covalent network structure? *Chlorine *Diamond *Graphite |
Chlorine
Chlorine is a diatomic molecule, so is a discrete covalent molecule. |
|

Which of these diagrams best represents the attractions that form the covalent bond holding the atoms together in the molecule? |

In a covalent bond, the atoms are held together as both nuclei are attracted to both the bonding electrons of the shared pair. |
|
|
How can the shape of methane (CH4) molecules be described? |

Tetrahedral
Methane forms tetrahedral molecules. |
|
|
What is the chemical formula for sodium bromide? |

|
|
|
What is the chemical formula for calcium phosphide? |

|
|
|
What is the chemical formula for calcium phosphide? |

The prefix 'tetra-' indicates that there are four chlorines in the formula. |
|
|
What is the chemical formula for dinitrogen trioxide? |

The prefix di- indicates 2 nitrogens in the formula and the prefix tri- indicates 3 oxygens in the formula. |
|
|
What is the chemical formula for ammonium sulfate? |

|
|
|
What is the chemical formula for iron(II) chloride? |

|
|
|
What is the chemical formula for copper(II) hydroxide? |

|
|
|
What is the chemical formula for calcium hydroxide? |

|

