![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
7 Cards in this Set
- Front
- Back
|
What are the names of the four quantum numbers of electrons in atoms? |
Principal Angular momentum Magnetic Spin |
|
|
What are the symbols for the four quantum numbers of electrons in atoms. |
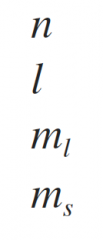
|
|
|
What are the properties for each of the four quantum numbers of electrons in atoms? |

|
|
|
What are the permitted values for each of the four quantum numbers of electrons in atoms? |

|
|
|
Give the name, symbol, permitted values and property for each of the four quantum numbers of electrons in atoms. |
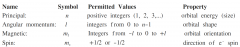
|
|
|
What does the exclusion principle state? |
That no two electrons in the same atom can have the same four quantum numbers! |
|
|
How many electrons can an atomic orbital hold? |
Each atomic orbital can hold a maximum of two electrons and they must have opposing spins! |

