![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
The force holding two atoms together is called a_____. |
Chemical bond |
|
|
|
Ionic bins occur between a ____ and a ___. |
Metal and nonmetal |
|
|
|
In a covalent bond, atoms_____electrons |
Share |
|
|
|
In an ionic bond there is a _____ of electrons from one atom to another. |
Transfer |
|
|
|
A ____ bond consists of one pair of electrons shared between atoms. |
Single covalent |
|
|
|
A ____bond consists of two pairs of electrons shared between atoms |
Double covalent |
|
|
|
A _____bond consists of three pairs of electrons shared between atoms |
Triple covalent |
|
|
|
When naming transition metals you need to include___ to represent the charge of the metal |
Roman numerals |
I,III, IV, VI |
|
|
What are the seven diatomic molecules? |
H2, O2, N2, F2, CL2, Br2, I2 |
|
|
|
What are the names and formulas of the five binary acids? |
HF - hydroflouric acid HCl - hydrochloric acid HBr - hydrobromic acid HI - hydroiodic acid H2S - hydrosulfiric acid |
|
|
|
If the polyatomic ion ends in -ate, the suffix of the acid should be___ |
-ic |
|
|
|
If the polyatomic ion ends in -ite, the suffix of the acid should be_____ |
-ous |
|
|
|
When naming tertiary acids, do you include the prefix hydro? |
No |
|
|
|
When naming binary acids, do you include the prefix hydro? |
Yes |
|
|
|
A solid that may be formed during a double replacement reaction involving aqueous solutions |
Solid precipitate |
|
|
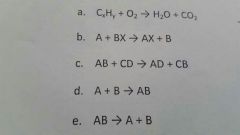
Classify the following reactions |
a. Combustion b. Single displacement (cationic) c. Double displacement d. Synthesis e. Decomposition |
|
|
|
Which types of reactions are essentially opposites of each other? |
Synthesis and decomposition |
|
|
|
What are three possible products/signs of a double displacement reaction? |
Solid precipitate, liquid h2o, or a gas |
|
|
|
Define spectator ions |
Ions that do not participate in the reaction. |
|
|
|
To determine if a single displacement reaction will occur refer to an____ |
Activity series |
|
|
|
To determine if a double displacement reaction will occur refer to a___. |
Solubility table |
|

