![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
Molecules are the smallest unit of ___________. Atoms are the smallest unit of ___________. |
Compounds. Elements. |
|
|
The atom is the smallest identifiable _________ of an element. |
UNIT |
|
|
An element cannot be broken down into simpler __________ , they can only be broken down into smaller parts of the same __________ . |
Substances. Substances. |
|
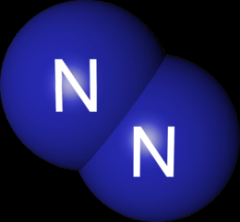
This is an example of what? |
A Diatomic molecule |
|
|
How many elements naturally occur? Therefore there are how many different kinds of (naturally occurring) atoms? |
91 91 |
|
|
Atoms are the ________ ________ of the physical world. |
Building blocks |
|
|
What does AMU stand for? |
Atomic mass unit |
|
|
1 proton or neutron is equal to how many amu's? |
1.0 atomic mass unit |
|
|
AMU=_________g |
1.67 X 10^-24 g |
|
|
What is the approximate mass of a Proton in grams? |
1.6726 X 10 ^ -24 g |

