![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
38 Cards in this Set
- Front
- Back
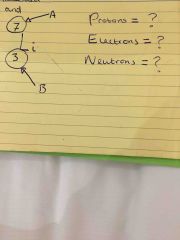
Complete the label diagram |
A = mass number B = atomic number Protons = (atomic number) = 3 Electrons = (same as protons) = 3 Neutrons = (mass number - atomic number) = 4 |
|
|
What is the relative charge of a proton, neutron and electron? |
P = +1 E = - 1 N = 0 |
|
|
What is the relative mass of a proton, electron and neutron? |
P = 1 E = 0 N = 1 |
|
|
What do periods represent in the periodic table? |
Periods represent the number shells in an atom. |
|
|
What do groups represent on the periodic table? |
Groups represent the number of electrons on the outer shell. |
|
|
Name the 3 separation techniques |
- filtration - crystallisation - distillation |
|
|
Why is filtration used as a separation technique? |
To separate an insoluble solid from soluble solids in a solvent. |
|
|
Name 3 properties about group 1 (alkali metals) on the periodic table |
- less dense than water - react with oxygen and water - soft to cut |
|
|
Alkali metals must be stored in oil so they don’t react with oxygen. True or False? |
True |
|
|
Define the word element |
A substance made up of only one kind of atom. |
|
|
What is an isotope? |
Different forms of the same element with the same amount of protons and electrons but different neutrons. |
|
|
What is an ion? |
Electronically charged particles formed when atoms lose or gain electrons. (Known as ionic bonding) |
|

Front (Term) |

Back (Definition) |
|
|
What is the charge for each group in the periodic table? |
- G1 = +1 - G2 = + 2 - G3 = + 3 - G4 = 0 - G5 = -3 - G6 = -2 - G7 = -1 |
|
|
How did John Dalton describe atoms at the start of 19th century? |
He described them as solid spheres and each element had a different sphere. |
|
|
By 1897, what did JJ Thompson conclude about John Daltons theory about the structure of an atom. |
He concluded that atoms weren’t solid spheres and an atom must contain even smaller particles such as electrons. |
|
|
Explain the method of filtration using an example of a sand water and salt solution |
The sand water and salt solution is poured into filter paper. The insoluble sand particles are left in the filter paper, whilst the the salt solution flows through the filter tunnel. |
|
|
When can crystallisation be used? |
To obtain a sample of pure salt. |
|
|
Describe the method of crystallisation in 4 steps. |
1. Pour the solution into an evaporating dish and gently heat the solution until some of the solvent is evaporated. 2. Once some of the solvent is evaporated, crystals will start to form. Remove the dish from the heat and let the solution cool. 3. The salt should start to form crystals as it becomes insoluble in the cold. 4. Filter the crystals out of the solution and leave them in a warm place to dry. |
|
|
What is simple distillation used for? |
Separation technique where pure liquid can be separated from a mixture of liquids. |
|
|
Describe the basic distillation method. |
The basic distillation method is done by boiling a solution. Once the liquid evaporates the vapour given of enters a condensers, (an outer glass tube with water flowing around it) the vapour is then cooled down into a liquid for collection in a receiving vessel. |
|
|
Name a characteristic of alkali metals boiling points |
The boiling point decreases going the group in the periodic table |
|
|
What happens to the reactivity going down group 1 on the periodic table. |
The reactivity increases going down the group. |
|
|
What happens when alkali metals react with water? |
They form a metal hydroxide |
|
|
What was JJ Thompson’s model called? |
The plum pudding model. |
|
|
What did Bohr’s nuclear model propose after Rutherford‘s theory? |
He proposed that electrons orbit the nucleus in fixed shells and each shell is a fixed distance from the nucleus. |
|
|
Which scientist provided evidence for neutral particles known as neutrons in the atom? |
James Chadwick |
|
|
And 1869 Dimitri Mendeleev published a periodic table, what was so significant about this periodic table? |
-He arranged the elements in order of atomic mass. - He also Left gaps in the table to make sure that the elements with similar properties stayed in the same groups some of these gaps indicated the existence of undiscovered elements. |
|
|
And 1869 Dimitri Mendeleev published a periodic table, what was so significant about this periodic table? |
-He arranged the elements in order of atomic mass. - He also Left gaps in the table to make sure that the elements with similar properties stayed in the same groups some of these gaps indicated the existence of undiscovered elements. |
|
|
What 3 things happen to the halogens, (group 7) as you go down the group? |
-they become less reactive -they have higher melting and boiling points -higher relative atomic masses |
|
|
What happens to alkali metals when they react with water? |
- The react vigorously to produce hydrogen gas a metal hydroxides. |
|
|
What happens to alkali metals when they react with water? |
- The react vigorously to produce hydrogen gas a metal hydroxides. |
|
|
What happens to alkali metals when they react with heated chlorine gas? |
They react vigorously with chlorine gas to form white metal chloride salts. |
|
|
What happens to alkali metals when they react with water? |
- The react vigorously to produce hydrogen gas and metal hydroxides. |
|
|
What happens to alkali metals when they react with heated chlorine gas? |
They react vigorously with chlorine gas to form white metal chloride salts. |
|
|
What happens to alkali metals when they react with oxygen? |
The react with oxygen to form a metal oxide. |
|
|
Name two things about noble gases which makes them different from any other element on the periodic table? |
They have eight electrons on their outer shell, (a full outer shell). Because their outer shell is stable they don’t lose or gain electrons which means they don’t react much. |
|
|
Complete the sentence: As you go down the group of noble gases the boiling point.... |
Increases |

