![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
71 Cards in this Set
- Front
- Back
|
Define chemistry |
the study of matter and the changes it undergoes |
|
|
what makes a property physical? |
if it can be observed without changing the substance into another substance |
|
|
what are some examples of physical properties? |
boiling point, density, mass, volume, color |
|
|
what makes a property chemical? |
if it can only be observed when a substance is changed into another substance |
|
|
what are some examples of chemical properties? |
flammability, corrosiveness, reactivity with acid |
|
|
what makes a property intrinsic? |
if it is independent of the amount of the substance that is present |
|
|
what are some examples of intrinsic properties? |
density, boiling point, color |
|
|
what makes a property extrinsic? |
if it is dependent upon the amount of the substance present |
|
|
what are some examples of extrinsic properties? |
volume (duh) energy and mass (vol in equation) |
|
|
what is a physical change? |
a change in matter that does not change the composition of a substance |
|
|
what is an example of a physical change? |
change in state, temperature, or volume |
|
|
what is a chemical change? |
change in matter that results in a new substance |
|
|
what is an example of a chemical change? |
combustion, oxidation, decomposition |
|
|
what are some signs of a chemical change? |
(unprompted) temperature change, color change, new smells, and sometimes bubbles |
|
|
what is a pure substance? |
matter that has distinct properties and a constant composition |
|
|
what are the two types of pure substances? |
compounds and elements |
|
|
what is an element/how is it identified? |
something that is comprised entirely of the same type of atom; cannot be decomposed into smaller substances; found on the periodic table |
|
|
what are the two types of elements? |
atomic and molecular |
|
|
what substance is written in the Formula AxByCz? |
a compound |
|
|
what are the two types of mixtures? |
homogeneous and heterogeneous |
|
|
what type of mixture is a solution? |
a solution is a homogeneous mixture |
|
|
what type of mixture is not uniform? |
a heterogeneous mixture |
|
|
what are the 6 methods of separation? |
1. filtration 2. evaporation 3. fractional crystallization 4. decantation 5. paper chromatography 6. distillation |
|
|
describe filtration |
separation of solid substances from liquids and solutions with a porous substance |
|
|
describe evaporization |
separation of liquids and solutions from solid substances by heat |
|
|
describe fractional crystalization |
separation of a solid dissolved in a liquid based on solubility |
|
|
describe decantation |
removing liquid from liquid-solid mixture by letting solids settle and pouring |
|
|
describe paper chromatogrophy |
separates substances based on differences in solubility in a solvent |
|
|
describe distillation |
separates liquids from solids in homogeneous mixtures on the basis of boiling point |
|
|
filtration is based on what property[s] |
particle size |
|
|
evaporation is based on what property[s] |
boiling point |
|
|
fractional crystallization is based on what property[s] |
solubility (and temperature) |
|
|
decantation is based on what property[s] |
density |
|
|
paper chromatogrophy is based on what property[s] |
solubility |
|
|
distillation is based on what property[s] |
boiling point |
|
|
what types of mixtures can be separated by filtration? |
heterogeneous mixtures |
|
|
what types of mixtures can be separated by evaporation? |
homogeneous mixtures |
|
|
what types of mixtures can be separated by fractional crystallization? |
homogeneous mixtures - solids dissolved in liquids |
|
|
what types of mixtures can be separated by decantation? |
heterogeneous mixtures |
|
|
what types of mixtures can be separated by paper chromatogrophy? |
homogeneous liquids |
|
|
what types of mixtures can be separated by distillation? |
homogeneous mixtures |
|
|
a gas has (a fixed/no fixed) volume and (a fixed/no fixed) shape? |
no fixed volume and no fixed shape |
|
|
a liquid has (a fixed/no fixed) volume and (a fixed/no fixed) shape? |
a fixed volume and no fixed shape |
|
|
a solid has (a fixed/no fixed) volume and (a fixed/no fixed) shape? |
a fixed volume and a fixed shape |
|
|
is the same volume of any constant substance most voluminous as a solid, liquid, or a gas? what about least voluminous? |
most as a gas, least as a solid |
|
|
how many uncertain digits are in one measurement? |
one |
|
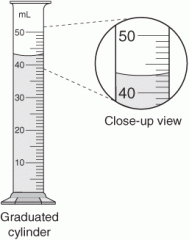
how many mL of water would you say are in this graduated cylinder? |
43.1 mL (approx. - around 43) |
|
|
in any given number or measurement, which digit is the uncertain one? |
the last one |
|
|
what is precision? |
proximity of several measurements to each other |
|
|
what is accuracy? |
proximity of a measurement to the true value of a quantity |
|
|
what would precision look like on a dart board? |
multiple arrows very close together (but not necessarily on the bulls eye |
|
|
what would accuracy look like on a dart board? |
an arrow very close to, or on, the bullseye |
|
|
what axis is the independent variable graphed on? |
the x-axis |
|
|
what axis is the dependent variable graphed on? |
the y-axis |
|
|
non-zero numbers are sometimes/always/never significant |
always |
|
|
captive zeroes are sometimes/always/never significant |
always (such as 505) |
|
|
leading zeroes are sometimes/always/never significant |
never |
|
|
trailing zeroes are sometimes/always/never significant |
sometimes (only if there is a decimal point somewhere in the number) |
|
|
how many significant figures are there in the number 163,582.764 |
9 (163,582.764) |
|
|
how many significant figures are there in the number 107? |
3 (107) |
|
|
how many significant figures are there in the number 000054.3? |
3 (000054.3) |
|
|
how many significant figures are there in the number 5.83 * 10^3 |
3 (5.83 * 10^3) |
|
|
how many significant figures are there in the number 5000? |
1 (5000) |
|
|
how many significant figures are there in the number 80000. |
5 (80000.) |
|
|
how many significant figures are there in the number 7890.00 |
6 (7890.00) |
|
|
how many significant figures are there in the number 0.0097 |
2 (0.0097) |
|
|
how many significant figures are there in the number 0.00400 |
3 (0.00400) decimal place only makes trailing zeroes significant - leading zeroes never are |
|
|
when addition or subtraction is performed answers are rounded to... |
the least significant decimal place |
|
|
when multiplication or division is performed answers are rounded to... |
the least number of significant figures |
|
|
what is always true about significant figures in conversion factors? |
conversion factors are infinitely significant |
|
|
what is the formula for density? |
D = m/v |

