![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
5 Cards in this Set
- Front
- Back
|
Word equation
|
Shows the words
Calcium + Oxygen -> Calcium oxide |
|
|
Symbolic equation
|
Shows the symbols
Ca + S -> CaS |
|
|
Coefficients
|
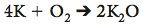
Numbers in front of each element showing the number of atoms of the element in a balanced equation
The 4 in front of potassium is a coefficient. If there is no number, Oxygen, then the coefficient is 1. |
|
|
Conservation of mass
|
Mass of reactants = mass of products
|
|
|
Balanced equation
|
The number of atoms of each element of the reactants = the number of atoms of each element of the products
Adjust coefficients to balance the equation |

