![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
34 Cards in this Set
- Front
- Back
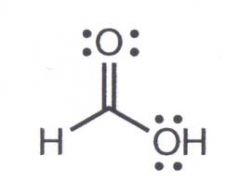
What is the common name
|
Formic Acid
|
|
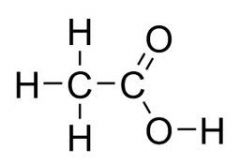
What is the common name
|
Acetic Acid
|
|
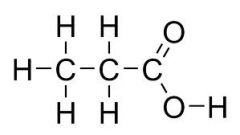
What is the common name
|
Propionic acid
|
|
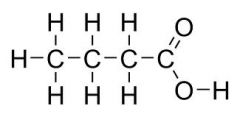
What is the common name?
|
Butyric acid
|
|

What is the common name?
|
Latic Acid
|
|
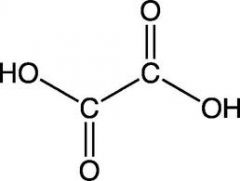
name this structure
systematic? common? |
S: EthaneDIoic acid
C: Oxalic acid |
|
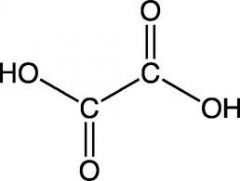
name this structure
systematic? common? |
S: EthaneDIoic acid
C: Oxalic acid |
|
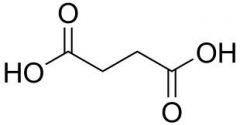
Name this structure
Systematic? Common? |
S: ButaneDioic acid
C: Succinic acid |
|
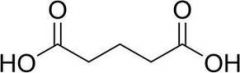
Name this structure
Systematic? Common? |
S: PentaneDioic acid
C: Glutaric acid |
|
|
How do you Name an Ester?
|
First name the alkyl group R in ester group -COOR
Ex: CH2CH3 = Ethyl Second name the parent acid EX: CH3COO=acetic acid Change -ic acid to -ate Ethyl acetate |
|
|
What form are low-molecular-weight unsubstituted amides?
|
Solids
|
|
|
What form is the simplest amide?
|
Formamide -HCONH2 liquid
|
|
|
Are the low-molecular-weight unsubstituted amides soluble in water? organic solvents?
|
yes
|
|
|
Which type of amides can form Hydrogen bonds to each other?
|
mono-substituted amides
Higher BP |
|
|
Which type of amides CAN'T form hydrogen bonds to each other?
|
Di-substituted amides
lower BP |
|
|
What is the difference between an amine and an amide?
|
Amides are bonded to a carbonyl group carbon. Amines are not.
|
|

What is this?
|
Benzoic acid
|
|
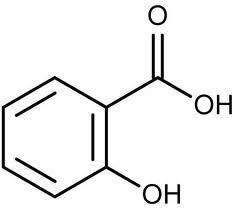
What is this?
|
Salicylic acid
|
|
|
What is the acid strength of alcohol?
Water? Phenol? |
10 -16
10 -7 10 -10 |
|
|
Carboxylic acids that are saturated and straight chain R groups are______________
|
Volatile liquids with strong pungent odors
|
|
|
Carboxylic acids up to four carbons are soluble in water
yes/no? |
yes
|
|
|
What form does Carboxylic acids with larger saturated R groups take?
|
Waxy, odorless solids
|
|
|
What is the reaction between an alcohol and a carboxylic acid to yield an ester plus water
|
Esterification
|
|
|
What are the reactants in esterification?
|
Carboxylic acid and alcohol
|
|
|
What are the products of esterification?
|
an ester and water
|
|
|
Is a catalyst needed in esterification? If yes, what is it?
|
Yes Strong acid like Sulfuric Acid or H+
|
|
|
How is an unsubstituted amide formed?
|
reaction of carboxylic acids with ammonia (NH3)
|
|
|
How is a substituted amide formed?
|
reactions between primary or secondary amines and carboxylic acids
|
|
|
For amide formation what other thing is needed in this reaction?
|
heat
|
|
|
How is an ester formed?
|
Carboxylic acid + alcohol w/ H+ catalyst
|
|
|
What is sliclylates?
|
esters of salicyclic acid
|
|
|
What is the products of Amide Hydrolysis?
|
Carboxylic Acid + Amine
|
|
|
What is the only thing that forms Dimers?
|
Carboxylic Acid
|
|
|
What is the products of Ester Hydrolysis?
|
Carboxylic Acid + Alcohol
|

