![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
41 Cards in this Set
- Front
- Back
|
What did the Scientist Dmitri Mendeleev do that made him famous?
|
He used the 50 known elements to create a chart, to arrange them into a table of element. But most importantly he had left some gaps in between some of the elements, for he had predicted that there were some remaining and undiscovered elements. Which were later reserved for the transition metal.
|
|
|
How is the Periodic Table arranged in terms of electrons and protons?
|
In all of the element, the number of protons and electrons are the same. As the number of protons and electrons increases the atomic number will do the same and will vary in different group
|
|
|
What is the trend of alkali metals?
|
As you go down the group the more reactive it becomes, eg. It becomes more violent in water, which it will produce Hydrogen gas and turn the water into an alkali solution.2Na + H₂O → 2NaOH + H₂It will also begin to have a lower density and melting point.
The outer electron is further fromnucleus as you go down the group and the atoms are larger |
|
|
Why do Alkali Metals forms Ionic Compounds with Non-metals?
|
Alkali metals are keen to lose their outer electron to form a 1+ ion, by doing this it will form an ionic bond which it will produce a white compound that can dissolve in water to form a colourless solution.However, it does not want to share its electrons, preventing covalent bonding.
|
|
|
What is the trend of Halogens?
|
As you go down the group 7, the elements will become less reactive, have a higher melting point and a higher boiling point.
|
|
|
What's the different between a halogen and halide ion?
|
Halogens are normally unreactive, whereas halide ion is very reactive
|
|
|
What is the Halogen displacement rule?
|
What is the Halogen displacement rule?
|
|
|
How does Halogens form Ionic Bonds with Metals?
|
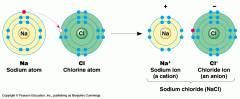
Halogen forms 1- ions called halides when they bond with metals. eg. Na+Cl-
|
|
|
Name the properties of a transition metal
|
They are...- good conductors of heat and electricity- very dense, strong and shiny- less reactive with water or oxygen- have a very high melting point (not including of mercury)
|
|
|
What is an industrial use of transition metals?
|
- Iron is used as a catalysis for the Haber process
- Manganese(IV) oxide is a good catalyst for the decomposition of hydrogen peroxide - Nickel is useful for turning oils into fats for making margarine |
|
|
Hard water causes scum and scales in kettles.
When scales reacts with an acid, what is the name of the gas produced? |
Carbon dioxide
|
|
|
What makes hard water, the way it is?
|
Hard water is when water has dissolved calcium and magnesium ions. It happens when rain falling on types of rock (eg. limestone and chalk) substances that can dissolve compound like magnesium sulfate and calcium sulfate both of which are soluble.
|
|
|
What causes temporary hardness in water?
|
It is caused by hydrogen carbonate ion,
HCO3-, in Ca(HCO3)2 |
|
|
What causes permanent hardness in water?
|
It is caused by dissolved calcium sulfate and other things
|
|
|
How to make hard water, soft?
|
By removing the magnesium and calcium ions. One way that this can be done by adding washing soda (sodium carbonate) to it, which the carbonate ion reacts calcium and magnesium ions to make an insoluble precipitation of calcium carbonate and magnesium carbonate. Ca²+ + CO3² → CaCO3 |
|
|
How to make temporary hard water, soft?
|
It can be removed by boiling it.
When it is heated, the calcium hydrogen carbonates to form calcium carbonate which is insoluble, as a result, it becomes limescale. |
|
|
What is good about hard water?
|
It is good for our teeth and bones due to the calcium and minerals in it. On top of this, it also decreases the chance of heart disease.
|
|
|
How is titration used to measure the hardness in water sample?
|
Add soap solution into the burette and add the water sample into the flask. Allow the soup solution to run into the water sample while stirring the flask. Until there is a good lather, that has been produced by the soap solution and hard water reacting
|
|
|
How is water treated to make tap water?
|
The water goes through a mesh screen to remove large bits such as twigs and sticks. Chemicals are then added to make remaining solid and microbes stick together and sink to the bottom. Water is then filtered through gravel beds to remove all the solids. It is then added with chlorine to kill off any harmful microbes left. |
|
|
What are the Cons and Pros of adding fluoride and chlorine?
|
Pro - Help reduce tooth decay - Prevent disease Cons - Increase the risk of cancer - increase the risk of heart disease - May cause tooth problems |
|
|
How does water filters reduce the hardness in water?
|
It has ions exchange resins
|
|
|
Why may water filters have particles of silver?
|
It can kill microbes in the water
|
|
|
What is a reversible reaction?
|
It is a reaction that can go both ways.
|
|
|
What is equilibrium?
|
It means when the certain amount of reactants and product will reach a certain balance and stay there.
|
|
|
How does changing the temperature of a reversible reaction alter the equilibrium?
|
When increasing the temperature the endothermic reaction will increase to use up the extra heat.
Whereas decreasing the temperature the exothermic reaction will increase to give out more heat |
|
|
How does changing the pressure of a reversible reaction alter the equilibrium?
|
When increasing the pressure the endothermic reaction it will encourage the reaction to produce less volume.
Whereas decreasing the pressure the exothermic reaction will encourage the reaction to produce more volume. |
|
|
How to make Ammonia?
|
Nitrogen and Hydrogen
N₂ + 3H₂ ↔ 2NH3 |
|
|
Draw a diagram of the Haber process?
|
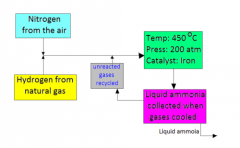
|
|
|
What are the conditions needed for the Haber process?
|
Pressure- 200 atmosphere
Temperature- 450C Catalysis- Iron |
|
|
How is ammonia separated from unreacted nitrogen and hydrogen in the Haber process
|
The produced ammonia will be cooled down and condense, to be in a liquid form
|
|
|
Why is an iron catalysis used in the Haber process?
|
It helps speed up the reaction and keep the cost of the Haber process down.
|
|
|
What is Alcohol functional group?
|
-OH The first three of this group is... Methanol CH3OH Ethanol C2H5OH Propanol C3H7OH |
|
|
What are the similar properties does alcohol have?
|
They are... - flammable, they burn in air to produce carbon dioxide and water. - can all dissolve in water to form a natural solution - react with sodium to give hydrogen and alkoxides |
|
|
What are Alcohol used for?
|
Ethanol is used for drinking, perfume and aftershave lotions, which is made with added oil to give the smell. It is also used as fuel in spirit burners and can be mixed with petrol in cars allowing less pollution |
|
|
What functional group Carboxylic acid is in?
|
- COOH The first three being... Methanoic acid CHCOOH Ethanoic acid CH3COOH Propanoic acid C2H5COOH |
|
|
What is Carboxylic acid used for?
|
Carboxylic acid with long chains of carbon atoms is used to make Soap and Detergents. They are also used in preparation in Esters. |
|
|
How is ethanoic acid made and what is the process called?
|
Ethanol and oxygen produced ethanolic acid, the process is called oxidation
|
|
|
What is the different between concentration and strength of an acid?
|
Concentration is the pH
Strength is how well the acid ionises in water |
|
|
What functional group is Ester part of?
|
- COO
Their names end in "-oate" The acid that forms the first part of the ester's name and the acid forms the second part eg. ethanol + ethanoic acid → ethyl ethanoate + water |
|
|
What are Esters used for?
|
They smell quite nice so they are used in perfumes. It is used to make flavouring and aromas, which can be used give off a taste or smell of a fruit eg. apples and oranges |
|
|
What are the two substances to form an ester?
|
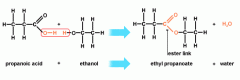
Alcohol + carboxylic acid → ester + water
With an acid catalysis often used (eg. sulfuric acid) |

