![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
50 Cards in this Set
- Front
- Back
|
Homogenous Mixtures |
Different particles are thoroughly mixed e.g. Salty water |
|
|
Heterogenous |
Particles in clumps of one kind e.g. Muddy water |
|
|
Element |
Made up of only one kind of atom, either single or molecule |
|
|
Compound |
Made of molecules containing two or more kinds of atoms |
|
|
Diatomic Elements |

H N O F Cl Br I |
|
|
Physical properties of a pure substance |
Characteristics of the substance that can be measured without a chemical reaction occurring E.g. Mass, volume, density Melting point/boiling point Heat capacity Brittleness |
|
|
Chemical characteristics of pure substances |
Characteristics of a substance that are related to the way in which it undergoes a chemical reaction E.g. Reactivity, acidity, flammability, half life, toxicity |
|
|
Extensive properties |
Depends of how much of the material |
|
|
Intensive properties |
Depends only what the material is |
|
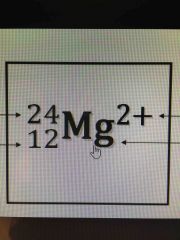
What's what ? |
24 is the mass number (neutrons + protons) 12 atomic number- protons 2+ is the charge ( lost 2 electrons) |
|
|
Atomic number |
Number of protons |
|
|
Mass number |
Number of protons + neutrons |
|
|
Ion |
An atom that has gained or lost and electron ( has charge ). Can be made of one atom or more joined together |
|
|
Monatomic ion |
Ion made of one atom |
|
|
Polyatomic ion |
More than one atom joined together |
|
|
Cations & anions |
Positive charge = cations Negative charge = anions |
|
|
What are some examples on chemical characteristics of pure substances? |
- Re-activity - Half life -Toxicity -Acidity - Flammability |
|
|
What are some physical characteristics of pure substances? |
Mass Volume Density melting point heat capacity brittleness
|
|
|
Types of chemical reactions |
- Combination reaction - Decomposition - Displacement (ionic) - Single/ double - Combustion |
|
|
Sig Figs X & /
+ & - |
X/ Round to the lowest number of sig figs
+- Round to decimal point |
|
|
Accuracy Definition |
How close a measured value is to the actual (true) value |
|
|
Precision |
How close the measured values are to each other |
|
|
What is a Solute? |
A solute dissolves in a solvent
|
|
|
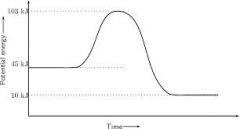
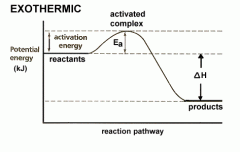
Exothermic |
Surroundings get hotter Releases energy
Products will have less energy than reactants
Enthalpy is negative |
|
|
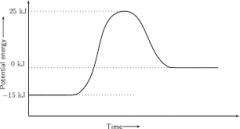
Endothermic |
Surroundings get cooler Absorbs energy
Products will have more energy than reactants
Enthalpy is positive |
|
|
|
|
|
|
|
|
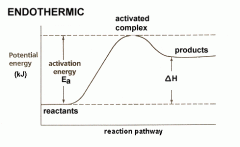
Activation Energy |
The energy needed to break the bonds in the reactant molecules, which then allows them to be rearranged and the reaction to proceed
- To get reaction started |
|
|
Change in Enthalpy (Triangle H) Formula |
Change in enthalpy is the enthalpy of the products take away the enthalpy of the reactants
= Hp - Hr |
|
|
Enthalpy |
Is the energy stored in bonds of molecules - When old bonds are formed |
|
|
Atomic Mass |
A weighted average of the masses for all the isotopes of a certain element
To find the atomic mass (Isotope A x %A) + (isotope B x %B) = RAM |
|
|
Quantitative |
describes a chemical reaction in terms of the amount of substances (quantities) |
|
|
Qualitative |
describes a chemical reaction in terms of what it does |
|
|
Electromagnetic Spectrum |
describes electromagnetic waves and the range of different wave lengths they can have - They can travel through empty space |
|
|
Electromagnetic Waves |
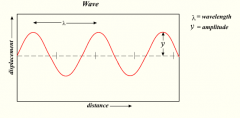
- can be absorbed, emitted or reflected by particle matter
|
|
|
Frequency |
pass between two points in one second |
|
|
Long and Small wave lengths |
- Large wave lengths = low energy - Small wave lengths = high energy
- The hotter the object gets the shorter the average wavelength |
|
|
Wave Particle Duality |
Wave–particle duality is the concept that every elementary particle or quantic entity exhibits the properties of not only particles, but also waves. |
|
|
c = l (Lamba) f |
Speed of light = wave length x frequency
- When f goes down l must go up and vis versa because C is a consent |
|
|
Photon |
Light particle - they can be absorbed or emitted by atoms as though they are particles |
|
|
Absorbtion and Emission spectra |
- Absorption spectra was produced by electrons absorbing photons as they move up from lower to high energy levels
- Emission spectra was produced by emitting electrons as they move down from a higher to lower energy levels |
|
|
Orbitals |
- 2 electons in each orbital -higher orbitals have higher energies - higher energy levels = larger orbitals
- At any point around the nucleus their is a certain probability that the electron will be there |
|
|
Shell Number ||| Electrons 1 2 2 8 3 18
Orbital Notation |
1 orbital ( 1s)
4 orbitals (2S, 2d 2d 2d)
9 Orbitals (3S, 3ppp, 3dddd) |
|
|
Atomic Radius |
Half the distance between two nuclei Across = smaller - You gain more protons increasing the positive charge (makes pull stronger)
Down = Larger - Adding electron shells |
|
|
Ionic Radius |
Radius of an Ion e.g Li+ is smaller than Li because is has lost an electron |
|
|
Ionic Radius Trends |
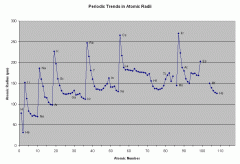
As you move across the ionic radius decreases with increase at 4/5
Down = increase |
|
|
Ionization Energy |
Ionization energy is the energy required to remove an electron from an atom or ion
The first ionization energy is the energy required to remove on electron from a neutral atom (always refers to valence electrons.
|
|
|
Ionization Energy Trends |
Across= increase Down = Deacrease
- How far electrons are from the nucleus (the further away the lesser the attraction and lower IE) - How many protons there are (more there is the greater the pull- higher IE) - How many electrons there are between the valence electron and the nuceus |
|
|
Electronegativity |
is a measure of the ability of an atom to attract the electrions in a bond
Across = Increase Down = Decrease |
|
|
Orbitals |
1 orbital (s1) |

