![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
Atom |
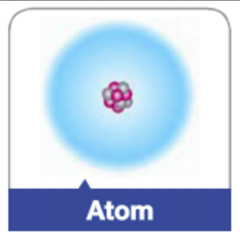
The fundamental building block of all materials; it consists of a cluster of protons and neutrons surrounded by a cloud of electrons. |
|
|
Atomic Model |
Scientists' representation of an atom determined by experiment and indirect observation. |
|
|
1) Atomic Number 2) Atomic Symbol 3)Atomic Mass (Mass Number) |
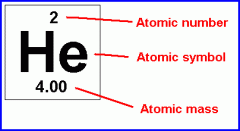
1) The number of protons in a nucleus; the atomic number determines what type of atom it is. 2) A short-hand notation for describing an atom; it consists of the chemical symbol, atomic number, and mass number. 3) The number of protons and neutrons is an atom. |
|
|
Compound |
A pure substance that is made up of two or more different types of atoms chemically joined. |
|
|
Crystal Lattice |
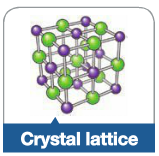
A grid-like structure of atom or irons where each particle is bonded to al of its neighbouring atoms. |
|
|
Electrons |
A small, negatively charged particles; clouds of electrons surround the nucleus of an atom. |
|
|
Electron Configuration |
The number of electrons in each of the electron shells of an atom |
|
|
Electron Shell |
Part of the electron cloud; it is a layer that surrounds the nucleus and can only hold a certain number of electrons. |
|
|
Element |
A substance made up of only one type of atom. |
|
|
Isotopes |
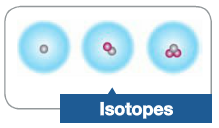
Atoms that have the same number of protons but a different number of neutrons in their nucleus. |

