![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
128 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Ionic bonding |
Between cation (+) and anions (-). The anions takes the electron from the cation (ions). Weak and strong. Low and high electronegativity |
|
|
|
Metallic bonding |
Between metals, a continuous passing of electrons. Weak and weak. Low and low electronegativity |
|
|
|
Covalent bonding |
Between non metals, fighting over electrons. Strong and strong. High and high electronegativity |
|
|
|
Octets rule |
Pairs are stable but 8 valence electrons is more stable |
|
|
|
BP 2 LP 0 |
Linear |
|
|
|
BP 2 LP 1 |
Bent |
|
|
|
BP 2 LP 2 |
Bent |
|
|
|
BP 2 LP 3 |
Linear |
|
|
|
BP 3 LP 0 |
Planar trigonal |
|
|
|
BP 3 LP 1 |
Trigonal pyramidal |
|
|
|
BP 3 LP 2 |
T-shaped |
|
|
|
BP 4 LP 0 |
Tetrahedral |
|
|
|
BP 4 LP 1 |
Seesaw |
|
|
|
BP 4 LP 2 |
Square planar |
|
|
|
BP 5 LP 0 |
Trigional bipyramidal |
|
|
|
BP 5 LP 1 |
Square pyramidal |
|
|
|
BP 6 LP 0 |
Octahedral |
|
|
|
Molecular compounds |
Made up of individual MOLECULES that stay together with IMF |
|
|
|
Metallic compounds |
A whole bunch of metal atoms |
|
|
|
Ionic compounds |
Positive and negative ions Metals and non metals |
|
|
|
Network compounds |
Made of covalent bonds, Ge, C, SiO and B. |
|
|
|
Boiling point |
When a liquid turns into a gas. Molecular cmpd: non-polar very low; polar low Metallic cmpd: pure medium low; alloy medium high Ionic cmpd: high Network cmpd: very high |
|
|
|
Melting point |
When a solid becomes a liquid. Molecular cmpd: non-polar very low; Polar low Metallic cmpd: pure medium low; alloy medium high Ionic cmpd: high Network cmpd: very high |
|
|
|
Freezing point |
When a liquid becomes a solid Molecular cmpd: non-polar very low; Polar lowMetallic cmpd: pure medium low; alloy medium high Ionic cmpd: high Network cmpd: very high |
|
|
|
Electron conductivity |
The ability to take an electron and transport it through the compound Molecular cmpd: noMetallic cmpd: yesIonic cmpd: yes/no (depends if dissolved in water) Network cmpd: no |
|
|
|
Dissolves in water / solubility in H2O |
Water is a molecular compound and polar which allows it to pull apart other polar compounds or fit in small compounds Molecular cmpd: non-polar yes/no (if small enough); Polar yesMetallic cmpd: noIonic cmpd:yes (no) (lots and little) Network cmpd: no |
|
|
|
Hardness |
When you rub them together which one scratches the other Molecular cmpd: non-polar very low hard 1-2; Polar low hard 2-3Metallic cmpd: pure medium low hard 4-5; alloy medium high hard 5-6Ionic cmpd: hard 7-8 Network cmpd: very hard 9-10 |
|
|
|
Intermolecular forces |
Are forces of attraction between: -neutral molecules of molecular cmpds -neutral diatomic molecules of diatomic elements -neutral atoms of non-metallic elements An indication of strength is boiling point.... The higher the boiling point the more IMFs A higher molar mass / size = a higher IMF |
|
|
|
Dipole-Dipole forces |
The attraction between molecules with a permanent separation of charges Only polar molecules have this |
|
|
|
Hydrogen bonding |
The attraction of a molecule containing hydrogen to another molecule A very strong dipole-dipole force When H is involved in a very polar bond with a very electronegative partner, H forms a region of particularly dense positive charge within a molecule H bonds with F, O or N. H's electron get pulled away from the nucleus and because H is so small the charge is more concentrated and there are no shells in the way 10x stronger that dipole-dipole |
|
|
|
London forces (dispersion forces) |
Due to temporary dipoles lining up and attracting the temporary or induced dipole of nearby molecules This is caused by the random motion of the electrons that cause temporary dipoles. This temporary separation of charges can cause nearby molecules to do the same More electrons = more possible dipole Bigger molecules have greater London forces Molar mass = more polarizable All molecules haven London forces |
|
|
|
Molar heat of fusion |
The amount of energy (heat) needed to change (melt) one mole of substance from a solid to liquid |
|
|
|
Molar heat of vaporization |
The amount of energy (heat) needed to change (boil) one mole of substance from liquid to a gas |
|
|
|
Heat (unit and variable) |
Q, KJ or J. (US- Calorie) A measurement of energy, two categories: kinetic energy and potential energy |
|
|
|
Kinetic energy |
Motion: vibrational, rotational and translational. Measured with a thermometer |
|
|
|
Potential energy |
The space between bonds. Measured with a calorimeter. A change of State and a chemical reaction are a positive potential energy change |
|
|
|
Potential energy equation |
Q=n delta H or ( +-)Q/n= delta H |
|
|
|
Kinetic energy equation |
Q=MC delta T |
|
|
|
Calorimeter |
Is used to measure potential energy change |
|
|
|
Thermometer |
Is used to measure the average kinetic energy |
|
|
|
Kinetic molecular theory |
Particle collision |
|
|
|
Temperature |
Average kinetic energy |
|
|
|
Delta H |
Enthalopy, which gives you a thermo-chemical equation. (Find it by a lab or research) |
|
|
|
Heat |
MCdeltaT =Q
*Q |
|
|
|
Heat capacity |
CM J/℃ The amount of energy to change it one ° |
|
|
|
Specific heat capacity |
C J/g℃ |
|
|
|
Hess' law type 1 |
delta H=(products)-(reactants) |
|
|
|
Hess' law type 2 |
Cancellation and addition |
|
|
|
Gibbs |
**crash course Big Bang Enthaply H: The universe likes exothermic reactions (where energy is released) Doesn't like endothermic reactions (where energy is absorbed) Entropy S: Distribution of energy (expansion, spreading out energy) The universe favors sending out and distributing energy ~so endothermic reactions occurs when entropy is stronger than enthalpy. Ex: exothermic reactions will occur if a solid turns into a gas because the gas particals will spread out more than the solid |
|
|
|
Gibbs free energy equation |
Delta G= delta H - T delta S (in Kelvin) Delta G= negative -> will occur Delta G=positive -> will not occur |
|
|
|
Kelvin |
0K = absolute zero = -273.15℃ ℃+273.15=K |
|
|
|
Standard temperature |
25℃ = 298.15K (Can) 0℃ = 273.15K (US) |
|
|
|
Entropy |
2nd law of thermodynamics Predictions based off particle distribution -> look at the states or the moles 1PCl3(g)+1Cl2(g)-> 1PCl5(g) 2 moles -> 1 mole States = no change >no distribution change Moles = decrease >there are fewer particles, decrease in entropy |
|
|
|
variables in R of Rxn: temperature |
Increase in temperature = increase of particle collision = increase of rate
Decrease in temperature = decrease in particle collision = decrease of rate *rule of thumb : and increase in 10℃ usually double the rate |
|
|
|
variables in R of Rxn: concentration |
Increase in concentration = increase in particle collision = increase in rate
Decrease in concentration = decrease in particle collision = decrease in rate The more chemical you react the faster the rate (usually) You must increase the concentration of the rate determining step to increase rate |
|
|
|
variables in R of Rxn: pressure |
Increase of pressure = increase in particle collision = increase in rate
Decrease of pressure = decrease in particle collision = decrease in rate
* two ways to affect pressure either more volume or less space Concentration regarding gas |
|
|
|
variables in R of Rxn: reaction mechanism -> Catalyst |
Increase in particle collision = increase in rate
* a catalyst is something that is put in to bang around but is formed again in the products |
|
|
|
Variables in R of Rxn: reaction mechanism -> inhibitor |
Increase in activation energy (Ea) = decrease in rate |
|
|
|
variables in R of Rxn: nature of the substance |
Stronger the bond = stronger Ea = slower reaction (decrease in rate)
Weaker bonds = lower Ea = faster reaction ( increase in rate),
States: (IMF) Gases faster (more K. E.) Liquid Solids ( less K. E.) * types of bonds and IMFs |
|
|
|
Enthalpy diagram (exothermic) |

***delta H per mole |
|
|
|
Enthalpy (endothermic) |
** delta H per mole |

|
|
|
Potential energy diagram (exothermic) |
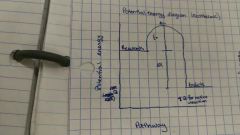
**delta H for entire reaction |
|
|
|
Potential energy diagram (endothermic) |
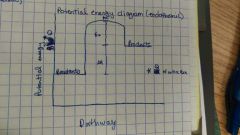
**delta H for entire reaction |
|
|
|
Activation energy (Ea) |
The minimum amount of energy it takes to achieve Ac, to change from a reactant to a product, the amount of energy the molecules need to collide with to break apart and from a new compound |
|
|
|
Activation complex (Ac) |
The point at which reactants turn into products. An unstable group of atoms formed at the instant of change over from reactants to products |
|
|
|
Boltzmann distribution diagram |
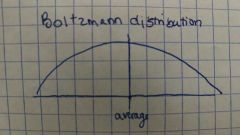
|
|
|
|
Rate determining step (RDS) |
The elementary step that has the highest Ea |
|
|
|
Elementary step |
The individual steps in a mechanism |
|
|
|
Intermediate product |
Something a product that is made then used |
|
|
|
Collision theory |
For a reaction to happen molecules must collide with one another |
|
|
|
Particle orientation |
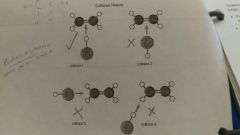
Is where the particles collide with one another * goes with collision theory |
|
|
|
Reaction rates |
Is a measure of how fast reactions occurs or how something changes during a given time period ** factors affect Rate. ** rate is usually measured with concentration |
|
|
|
The three types of reaction rate |
Modified: start and end (average) Instantaneous: measured at any time (singular) True: calculating the curse |
|
|
|
Reaction rates depends on.... |
Surface area Temperature Concentration Amount of kinetic energy Bonds Pressure Reaction mechanism |
|
|
|
Homogeneous catalyst |
The reagent and catalysts are in the same phase, it looks the same as what it's in |
|
|
|
Heterogeneous |
The catalyst is in a separate phase from the reactant. You can't see it, it's not the same |
|
|
|
Mechanism |
Which consists of multiple elementary steps |
|
|
|
Proportionality statement |
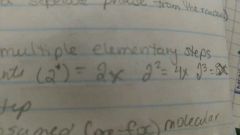
Orders and exponents |
|
|
|
Elementary |
Consists of one elementary step |
|
|
|
Molecularity |
The amount of moles consumed (Prefix) molecular : uni, di, tri, quad, quint, sex, Sept, octo, novem, deca N2O5->NO2+NO3 (unimolecular) NO+NO3->2 NO2 (bimolecular) C2H2 + 2HCl-> C2H4Cl2 (trimolecular) |
|
|
|
The three steps of rates of reactions |
1. Determine if a reaction will occur (Gibbs) 2. What variables will increase or decrease the reaction rate? (Knowledge) 3. Development a rate law (math equation) to predict future rates |
|
|
|
Rate |
The time needed for a change to occur |
|
|
|
K |
Rate constant |
|
|
|
Powers for rate of reaction law |
How much the variables affect the rate 0= equally 1= some change 2 = double change 3 = triple change |
|
|
|
Equilibrium |
It is possible for products to collide with enough force to revert back to reactants It does not refer to the amount but equal amounts moving back and fort An equal rare going back and forth You can only have equilibrium if you close the system To know is equilibrium is happening: Colour will appear to no longer be the same All measurable Ganges will appear to be no longer changing If there is reactants and products present it may never be stable |
|
|
|
Equilibrium law |
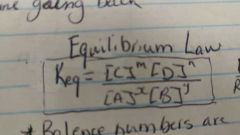
Products/reactants Balancing numbers are the powers when at equilibrium |
|
|
|
Water |
Self ionizing Has no concentration PH of 7 POH of 7 Kw= 1.0x10-14 |
Equilibrium and acid / base |
|
|
Acid |
A molecule or other entity that cab donate a protein or accept an electron pair in a reaction Can neutralize bases (alkali's) Dissolves some metals Turns blue litmus red Typically corrosive Sour taste Liquid** |
|
|
|
Strong acid |
Will completely ionize. The acid will break apart in water into the ions it is made up of Single arrow |
|
|
|
Weak acid |
Is only partially ionized. Only some molecules become in a but most stay as molecules
Equilibrium double arrow |
|
|
|
Bronsted Lowry Acid |
Substance that donates a proton. (H+) |
|
|
|
Lewis |
A chemical that donates protons or accepts electrons |
|
|
|
Arrhenius |
A substance that ionizes and dissociates in an aqueous solution to produce hydrogen ions (H+) |
|
|
|
Bases |
A molecule that will accept a proton or give up an electron pair Taste bitter Will change red litmus blue Aqueous solution can conduct electricity Reacts with acids to form salt and water Increase concentration of OH- in water Base solution has an PH >7 |
|
|
|
Strong base |
Fully ionizes in water and has OH- ions and a metal ions Single arrow |
|
|
|
Weak base |
Does not completely ionize in water, creates OH- ions in water Equilibrium double arrow |
|
|
|
Arrhenius base |
Substance which produces hydroxide ions |
|
|
|
Bronsted Lowry base |
Substance that are proton acceptors (which created hydroxide) |
|
|
|
Neutralization reactions |
Acid + bases (<)-> water and salt Acid + ammonia -> ammonium salt |
|
|
|
Conjugate base |
A product made from an acid which was an reactant |
|
|
|
Conjugate acid |
A reactant acid made from a base that was a reactant |
|
|
|
Amphoteric |
Duel roles is is an acid or a base
Ex: H2O -> OH- + H3O |
|
|
|
Change in concentration in equilibrium |

Water (liquids) and solids do not have a concentration |
|
|
|
Change in temperature equilibrium |

|
|
|
|
Change in pressure equilibrium |

When pressure decrease = moles increase When pressure increase = moles decrease |
|
|
|
Catalyst on equilibrium |
Has not effect |
|
|
|
Intermolecular bonding (ionic and covalent) |
The chemical binding that holds atoms together within a molecule of a compound (covalent and ionic)
|
|
|
|
Intermolecular bonding |
Vanderwal forces: London forces, dipole-dipole, hydrogen |
|
|
|
Bigger Ksp (closer to 1) |
Dissolves more |
|
|
|
Smaller Ksp (farther away from 1) |
Dissolves less |
|
|
|
Dissolving equation |
Solute (l, s, g,) -> <- solute (aq) |
|
|
|
What happens when temperature changes |
Changes |
Ksp |
|
|
Common ion |
Goes into the initial of the other equation |
|
|
|
Where does equilibrium take place |
In saturated water |
|
|
|
Saturated solution |
Max amount of chemical in the solution |
|
|
|
I. P. Test |
Ion product test, to determine if a solid is made |
|
|
|
What is a redox reaction |
A reaction that has a change in oxidation numbers |
|
|
|
Oxidation number |
If you lose or gain an electronic. |
|
|
|
Ionic oxidation numbers |
The same as the charge |
|
|
|
Molecular oxidation numbers |
It's about who has control The addition of oxidation numbers equal zero |
|
|
|
Poly atomic oxidation numbers |
Addition of oxidation numbers equal the charge |
|
|
|
Oxidation number of an element |
0 |
|
|
|
No change in oxidation numbers = |
No change in electroactivity = no redox, no oxidation and no reduction |
|
|
|
G.E.R. |
Gain electron reduction = oxidation agent (which causes L.E.O) |
|
|
|
L.E.O. |
Lose electron oxidation = reducing agent (which causes G.E.R.) |
|
|
|
Specific heat capacity of water |
4.184 |
|
|
|
Specific heat capacity of styrofoam |
1.3 |
|
|
|
Ksp |
Solubility product Saturation limit for a low soluble compound |
|

