![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
Mass Percent
|

-
|
|
|
Convert Equilibrium Constant
|

-
|
|
|
Half-Life from rate constant
|

-
|
|
|
Molality
|

-
|
|
|
Molarity
|

-
|
|
|
Mole Fraction
|

-
|
|
|
Osmotic Pressure
|

-
|
|
|
Solvent Vapor Pressure
|

-
|
|
|
Radioactive decay formulas for time
|

-
|
|
|
Gibbs Equations
|

H and S must both be kJ/mol
Faradays is 96,485 C/mol or J/V-mol |
|
|
Relate coulombs to amps and volts
|

-
|
|
|
K from delta G
|
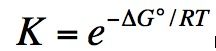
-
|

