![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
14 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Solution |
A mixture of a solid dissolved in a liquid |
|
|
|
Soluble |
A solid that dissolves |
|
|
|
Solute |
The dissolved solid in a solution |
|
|
|
Solvent |
The liquid part of a solution |
|
|
|
Filtration |
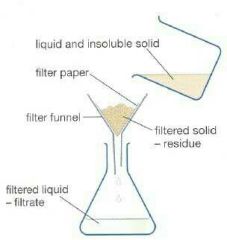
A method used to separate insoluble solids from solutions |
|
|
|
Crystallization |
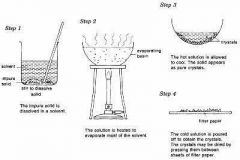
Is the process in which crystals are formed either from something that has been melted or from a solution. |
|
|
|
Simple distillation |
Simple distillation is used to separate a solvent from a solution |
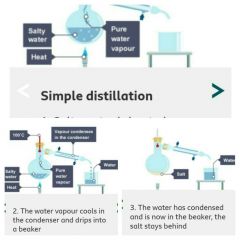
1. Salty water is heated 2. Water vapor cools in the condenser and drips into beaker 3. The water has condensed and is now in the beaker, the salt stays behind |
|
|
Fractional distillation |
Fractional distillation is used to separate different liquids from a mixture of liquids. |
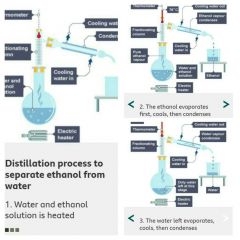
|
|
|
Chromatography |
Is a method used to separate mixtures and can give information to help identify substances.
Paper chromatography is used to separate mixtures of soluble substances. These are often coloured substances such as food colourings, inks, dyes or plant pigments. Distance traveled by substance ÷ Distance traveled by solvent |
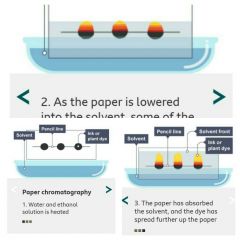
2. As the paper is lowered into the solvent, some of the dye spreads up the paper.
|
|
|
Element |
Element can be a solid, liquid or gas. The smallest particle of such an element is an atom. Atoms are made up of protons, neutrons, and electrons. |
|
|
|
Compound |
Something that is composed of two or more separate elements; a mixture. Water, for example, is a compound having two hydrogen atoms and one oxygen atom per molecule.
Pure elements and compounds melt at a specific temperatures melting point and boiling point data can be used to distinguish pure substances from a mixture. |
|
|
|
Mixture |
A substance made by mixing other substances together. |
|
|
|
Pure Substance |
A single element or compound not mixed with any other substances. |
|
|
|
Formulation |
A mixture that has been designed as a useful product many products have complex mixtures in which each chemical has a particular purpose.
They are made by mixing the conponents in carefully measured quantities to ensure that the product has the required properties. E.g fuels, cleaning agents,paints,medicines, fertilisers and foods. |
|

