![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
70 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Ionic Bond
|
metals and non metals
electrons transferred (from metal to nonmetal) their oppositely charged ions attract each other, lowering their overall potential energy |
|
|
|
Covalent Bonds
|
nonmetals and nonmetals
electrons shared between both nonmetals the shared electrons react with the nuclei of both atoms, lowering their potential energy |
|
|
|
Metallic Bonds
|
metals and metals
electrons pooled, 'lattice pool' the electrons aren't localized over a single atom, but delocalized over the entire metal; the positively charged metal atoms are then attracted to the sea of electrons holding it together |
|
|
|
Why do chemical bonds form?
|
because they lower the potential energy between the charged particles that compose atoms
coulombs law states that when two atoms approach each other the electrons of one atom become attracted to the nucleus of the other |
|
|
|
Metals
|
tend to have low ionization energies, meaning that their electrons are easy to remove
|
|
|
|
Nonmetals
|
tend to have negative electron affinities, meaning that they readily gain electrons
|
|
|
|
Cation
|
metal (loss of electrons)
|
|
|
|
Anion
|
nonmetals (gain of electrons)
|
|
|
|
Lewis Structure
|
represents valence electrons of an element with dots
|
|
|
|
Octet
|
an element having 8 valence electrons, completing their outermost shell
noble gasses many elements bond in order to complete their octets, becoming more stable |
|
|
|
Octet Rule Exceptions
|
Too few:
Hydrogen (1 electron, 1 bond = 2 valence) Beryllium (2 electrons, 2 bonds = 4 valence) Boron (3 electrons, 3 bonds = 6 valence) Expanded Octets: Sulfur (5 electrons, 5 bonds = 10 valence) Phosphorus (6 electrons, 6 bonds = 12 valence) |
|
|
|
Chemical Bond
|
the sharing or transfer of electrons to attain stable electron configurations for the bonding atoms
|
|
|
|
Lattice Energy
|
the energy associated with forming a crystalline lattice of alternating cations and anions from gaseous ions
the easiest way to calculate lattice energy is with the born-haber cycle |
|
|
|
Sublimation
|
going from a solid to a gas
|
|
|
|
Dissociation
|
separating of a compound
|
|
|
|
Born-Haber Cycle
|
a hypothetical series of steps that represents the formation of an ionic compound from its constituent elements
the steps are chosen so that each step is known except for the last one, which is lattice energy the change in enthalpy is also known (ΔH final) |
|
|
|
Born-Haber Cycle formula
|
ΔH final= ΔH1+ΔH2+ΔH3+ΔH4+ΔH5
change in enthalpy= (sublimation + bond energy + ionization energy + electron affinity + lattice energy/formation energy) *for bond energy don't forget to divide if needed* |
|
|
|
Trend in Atomic Radius
|
increases as you go down and to the left of the periodic table
|
|
|
|
Coulomb's Lattice Energy Law
|
E = K(Qpos + Qneg)/r
E: potential energy Qpos: charge of cation Qneg: charge of anion R: atomic radius/distance between ions K: proportionality constant (the more negative a lattice energy, the stronger it is) |
|
|
|
Lattice Energy Trends with Radius
|
as you go down a column, the radius increases, meaning that the lattice energy will be less because the ions aren't as close and do not release as much energy when the lattice forms
|
|
|
|
Lattice Energy Trends with Charge
|
as the charges of the ions increase, the lattice energy increases
charge affects lattice energy more than radius multiply the charges of each element involved, and the one with the largest negative total will have the strongest lattice energy |
|
|
|
Lattice Energy and Melting Points
|
higher lattice energy, the higher the melting point
|
|
|
|
Exothermic and Endothermic Properties of Lattice Energy
|
lattice energy becomes more exothermic (more negative) with increasing charge, and more exothermic with closer radii
lattice energy becomes more endothermic (less negative) with decreasing charge, and more endothermic with increasing radius |
|
|
|
Exothermic
|
gives off heat, transfers thermal energy from the system to the surroundings
loss of energy |
|
|
|
Endothermic
|
needs heat supplied into system from the surroundings to begin
increase in energy |
|
|
|
Double Bond
|
two atoms share two pairs of electrons
|
|
|
|
Triple Bond
|
two atoms share three pairs of electrons
|
|
|
|
Covalent bonds are highly...
|
directional, the attraction between the two bonded atoms is due to the sharing of electron pairs in the space between them
the interactions are between the molecules, aka intermolecular forces |
|
|
|
Ionic bonds are...
|
nondirectional and hold together an entire array of ions
|
|
|
|
Molecular Compounds vs Ionic Compounds
|
molecular compounds have lower melting and boiling points than ionic compounds
|
|
|
|
Problem with Lewis Structures Regarding Electron Sharing
|
it shows that in covalent bonds the electrons appear to be equally shared, but that's not the case. Each element has a different electronegativity and therefor a stronger or weaker pull for those electrons
|
|
|
|
Trend in Electronegativity
|
increases as you go up and to the right on the periodic table, with F being the strongest electronegative
|
|
|
|
Electronegativities
|
the element further up and to the right is more electronegative, symbolized by 𝛿- meaning the pull of electrons will tend towards this element (higher electron density). Bears a partial negative charge.
the element not as far to the right and up is more electropositive, symbolized by 𝛿+, the pull of electrons is not in their favor (lower electron density). Bears a partial positive charge. |
|
|
|
Polar covalent bonds
|
when elements of two different electronegativites bond together, giving an unequal sharing of electrons
has a positive and negative pole |
|
|
|
Determining Bond Types by their Electronegativity
|
(0 - 0.4) → small electroneg diff → covalent bond
(0.4 - 2.0) → intermediate electroneg diff → polar covalent bond (2.0+) → large electroneg diff → ionic bond To determine the difference, subtract the more electronegative atom to the less electronegative atom and compare the value. |
|
|
|
Electronegativity Related to Atomic Size
|
the larger the atom, the less ability it has to attract electrons to itself in a chemical bond
|
|
|
|
How to calculate percent ionic character
|
___pm (4.80D/100pm) = x (measured D/x)(100)= % ionic
|
Xx
|
|
|
Percent Ionic Character
|
the ratio of a bond's actual dipole moment to the dipole moment it would have if electrons were 100% transferred from one atom to another
in general, bonds with 50%+ ionic character are ionic bonds |
|
|
|
How to write a Lewis Structure
|
1. more electronegative elements go on the ends, the least electronegative goes in the center
2. calculate total number of electrons in compound (if has neg charge add 1 electron and if pos charge subtract and electron) 3. Fill up the atoms with electrons, aka dots, and if you have too many electrons, create more bonds until octets are full, some will have more or less than a complete octet |
|
|
|
Resonance Structures
|
represents all the possible representations of a compound with double arrows in-between them
some are more favorable structures, the one with the least formal charges will be the most favorable |
|
|
|
Resonance Hybrid
|
the actual structure of a compound with multiple structures is more intermediate with the positions of electrons in each different structure
Hybrids are represented with a dashed line over the bonds where different electrons are shared/bonded. One end may have a double bond and another doesn't and they intermediately switch between sides the bond is located. |
|
|
|
Resonance Stabilization
|
the delocalizing of electrons, by having intermediate positions for these electrons, creating them more stable
|
|
|
|
Formal charge
|
a fictitious charge assigned to atoms in a lews structure
represents the charge it would have if all bonding electrons were shared equally between the bonded atoms |
|
|
|
How to calculate formal charge
|
# of electrons it's supposed to have (group number) - actual amount of electrons directly touching it
|
|
|
|
Rules for formal charges
|
1. the sum of all neutral charges on a molecule must be zero
2. the sum of all the formal charges of an ion must equal that charge 3. small (or zero) charges on individual atoms are better than larger ones 4. when formal charges cannot be avoided, the negative formal charge should reside on the most electronegative atom |
|
|
|
Odd-Electron Species
|
have an odd number of electrons when bonded
the N on NO2 can have 7 |
|
|
|
Incomplete Octets
|
don't fill up to exactly 8 electrons when bonded
Hydrogen (1 electron, 1 bond = 2 valence) Beryllium (2 electrons, 2 bonds = 4 valence) Boron (3 electrons, 3 bonds = 6 valence) |
|
|
|
Expanded Octets
|
have too many initial electrons and therefore can make more than 4 bonds
Sulfur (5 electrons, 5 bonds = 10 valence) Phosphorus (6 electrons, 6 bonds = 12 valence) |
|
|
|
Bond Energies
|
the energy required to break one mole of the bond in the gas phase
they are always positive because it takes energy to break a bond compounds with stronger bonds tend to be more stable, and less chemically reactive |
|
|
|
Bond Lengths and Strengths
|
triple / double / single
shorter → longer stronger → weaker |
|
|
|
Bond Energies to Estimate Enthalpy Changes
|
average bond energies are used to estimate the enthalpy change of a reaction
Formula: ΔHrxn= (sum of bonds broken) -( sum of bonds formed) broken bonds = positive formed bonds= negative |
|
|
|
The 'thermic' of a reaction
|
exothermic- when weak bonds break and strong bonds forms
endothermic- when strong bonds break and weak bonds form |
|
|
|
Valence Shell Electron Pair Repulsion (VSEPR) Theory
|
based on the ides that electron groups, ('lone pairs', single-triple bonds and singe electrons) repel one another through coulombic forces
the repulsions between electron groups on interior atoms of a molecule determine the geometry of the molecule |
|
|
|
VESEPR Trends
|
increased VSEPR repulsion from lone pairs reduces bond angles
lp vs lp repulsion > lp vs bp repulsion > bp vs bp repulsion |
|
|
|
Predicting Molecular Geometry
|
1. draw lewis structure
2. count number of bonds and lone pairs on central atom 3. use VSEPR to predict the geometry |
|
|
|
Electron (VSEPR) Geometry
|
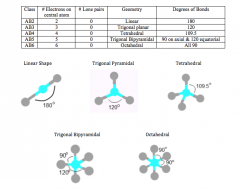
count all electron bonding pairs (double bonds count as one)
the lone pairs do not influence |
|
|
|
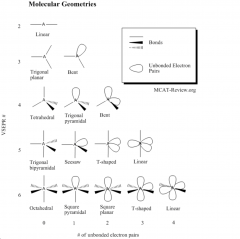
Molecular Geometry
|
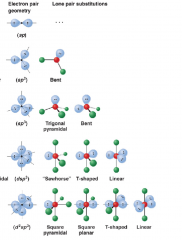
the non bonding, lone pairs will influence the shape now
|
|
|
|
How to Represent Molecular Geometries on Paper
|
hatched wedge: bond going into the page
solid wedge: bond coming out of page straight line: bond in plane of paper |
|
|
|
Determining Polar Molecules
|
At least 1 polar covalent bond + asymmetrical shape
if it is a non polar molecule as a whole, it will not be polar |
|
|
|
Colors of Polar Molecules in 3D Representations
|
𝛿- redish-yellowish color
𝛿+ blueish-greenish color use molecular geometry to know shape |
|
|
|
Dipole Moment
|
dipoles point to the more electronegative atom
no net dipole moment: sum of dipoles = zero (do not point in universal direction) dipole moment: point in a universal direction |
|
|
|
Hybridization
|
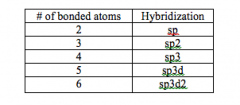
mixing of two or more atomic orbitals to form a new set of hybrid orbitals
|
|
|
|
Sigma Bonds
|
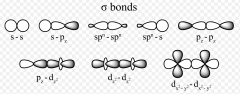
directly connect
electron density is between two atoms single bonds tip to tip laying down, with two ends touching formation |
|
|
|
Pi Bonds
|
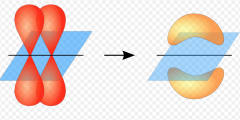
electron density above and below plane of nuclei
single and double bonds side by side standing up formation with each tip overlapping |
|
|
|
Single, Double and Triple Bonds
|
Single: 1 sigma
Double: 1 sigma, 1 pi Triple: 1 sigma, 2 pi |
|
|
|
Molecular Orbital Theory
|
bonds are formed from interaction of atomic orbitals to form molecular orbitals
|
|
|
|
Antibonding Molecular Orbitals
|
higher energy and lower stability
symbolized by sigma* |
|
|
|
Bonding Molecular Orbitals
|
lower energy and greater stability
symbolized by sigma |
|
|
|
Bond Order Formula
|
(1/2)(electrons in bonding - electrons in antibonding)
lower bond order = less stable |
|
|
|
How to fill in molecular orbital charts
|
fill in electrons on left and right following all the filling rules (2s first, then 2p, etc)
then fill in center molecule with bonding (at bottom) electrons going in before anti bonding (at top) (ADD THE TWO ELECTRONS ON BOTH SIDES TO FILL IN CENTER) |
|

