![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
79 Cards in this Set
- Front
- Back
|
Matter
|
Anything that has mass and takes up space and basically makes up the universe.
|
|
|
Solid
|
has definite shape and volume
|
|
|
Liquid
|
has definite volume, changeable shape
|
|
|
Gas
|
has changeable shape and volume
|
|
|
Energy
|
The capacity to do work (put matter into motion)
|
|
|
Kinetic
|
energy in action
|
|
|
Potential –
|
energy of position; stored (inactive)
|
|
|
Chemical
|
– stored in the bonds of chemical substances
|
|
|
Electrical
|
– results from the movement of charged particles
|
|
|
Mechanical
|
– directly involved in moving matter
|
|
|
Radiant or electromagnetic –
|
energy traveling in waves (i.e., visible light, ultraviolet light, and X rays)
|
|
|
Energy is easily converted from one form to another
Ture or false? |
True
|
|
|
During conversion, some energy is “lost” as sound.
true or false |
False- energy is lost through Heat
|
|
|
The composition of matter is comes in what two basic properity?
|
Atoms and elements
|
|
|
Elements
|
– unique substances that cannot be broken down by ordinary chemical means
|
|
|
Atoms –
|
more-or-less identical building blocks for each element
|
|
|
Atomic symbol
|
– one- or two-letter chemical shorthand for each element
|
|
|
elements as a whole have similar physical and chemical properties
True or false? |
False: Each element has unique physical and chemical properties
|
|
|
Physical properties
|
– those detected with our senses
|
|
|
Chemical properties
|
– pertain to the way atoms interact with one another
|
|
|
what are the 4 Major Elements of the Human Body?
|
Oxygen (O)
Carbon (C) Hydrogen (H) Nitrogen (N) |
|
|
What elements make up 3.9% True or False?
|
Lesser elements
|
|
|
Lesser elements make up 3.9% of the body and include:
|
Calcium (Ca), phosphorus (P), potassium (K), sulfur (S), sodium (Na), chlorine (Cl), magnesium (Mg), iodine (I), and iron (Fe)
|
|
|
what elements make up less than 0.01% of the body and are required in minute amounts, and are found as part of enzymes
|
Trace elements
|
|
|
Atomic Structure:
The nucleus consists of |
neutrons and protons
|
|
|
Atomic Structure: These have no charge and a mass of one atomic mass unit (amu)
|
Neutrons–
|
|
|
Atomic Structure: these have a positive charge and a mass of 1 amu
|
Protons
|
|
|
Electrons are found orbiting the nucleus. true or false?
|
True
|
|
|
These have a negative charge and 1/2000 the mass of a proton (0 amu)
|
Electrons –
|
|
|
Name 2 Models of the Atom
|
Planetary Model and
Orbital Model – |
|
|
what atom model shows that electrons move around the nucleus in fixed, circular orbits
|
Planetary Model –
|
|
|
What atom model shows regions around the nucleus in which electrons are most likely to be found
|
Orbital Model –
|
|
|
Molecule –
|
two or more atoms held together by chemical bonds
|
|
|
Compound –
|
two or more different kinds of atoms chemically bonded together
|
|
|
two or more components physically intermixed (not chemically bonded)
|
Mixtures –
|
|
|
Hydrogen
|
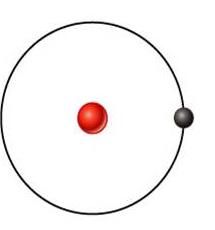
what's this element?
|
|
|
hellum (He)
|
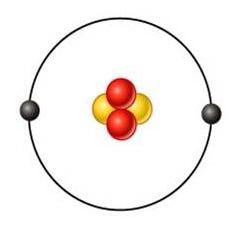
what's this element?
|
|
|
lithium (Li)
|
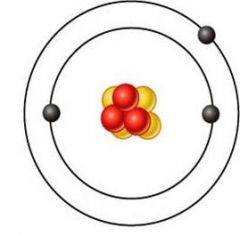
what's this element?
|
|
|
deutrium
|
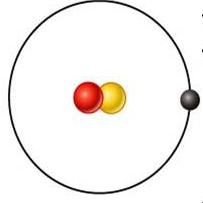
what is this element?
|
|
|
Tritium
|
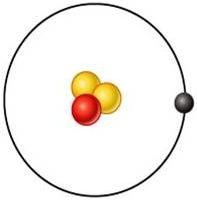
What is this element?
|
|
|
– substance present in greatest amount
|
Solvent
|
|
|
substance(s) present in smaller amounts
|
Solute –
|
|
|
homogeneous mixtures of components
|
Solutions –
|
|
|
Concentration of Solutions is measured by?
|
1. Percent, or parts per 100 parts
2. Molarity, or moles per liter (M) |
|
|
A mole of an element or compound is equal to its atomic or molecular weight (sum of atomic weights) in liters
true or false? |
False, its measured in grams
|
|
|
name 2 heterogeneous mixtures whose solutes do not settle out
|
Suspensions and
Colloids, or emulsions, |
|
|
Colloids, or emulsions, and Suspensions are heterogeneous mixtures with visible solutes that tend to settle out
True or false? |
True
|
|
|
True or false:
Only chemical bonding takes place in mixtures |
False NO chemical bonding takes place in mixtures
|
|
|
T or F: Most mixtures can be separated by physical means
|
True All compounds are homogeneous
|
|
|
T or F: Mixtures are heterogeneous but not homogeneous
|
False: Mixtures can be BOTH heterogeneous or homogeneous
|
|
|
T o F: Compounds cannot be separated by physical means
|
True
|
|
|
T or F: All compounds are homogeneous
|
True
|
|
|
T or F: Bonds surround the nucleus of an atom
|
false: Electron shells, or energy levels, surround the nucleus of an atom
|
|
|
T or F: Electron shells, or energy levels are formed using the electrons in the outermost energy level
|
false: Bonds are formed using the electrons in the outermost energy level
|
|
|
what is the outermost energy level containing chemically active electrons
|
a Valence shell –
|
|
|
a single Covalent bond
|
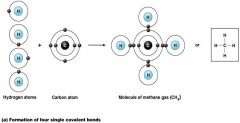
what kind of bond is this?
|
|
|
a double Covalent bond
|
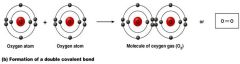
what kind of bond is this?
|
|
|
a triple Covalent bond
|
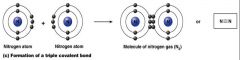
what kind of bond is this?
|
|
|
formation of an ionic bond
|
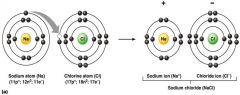
what does this picture represent?
|
|
|
Valence shell –
|
outermost energy level containing chemically active electrons
|
|
|
except for the first shell which is full with two electrons, atoms interact in a manner to have eight electrons in their valence shell
|
Octet rule –
|
|
|
Name the three Types of Chemical Bonds
|
Ionic
Covalent Hydrogen |
|
|
Ions, Anions and Cations are examples of?
|
Ionic Bonds
|
|
|
Ions are ?
|
charged atoms resulting from the gain or loss of electrons
|
|
|
Anions have ?
|
gained one or more electrons
|
|
|
Cations have ?
|
lost one or more electrons
|
|
|
t or F: Ionic bonds form between atoms by the transfer of one or more electrons?
|
true
|
|
|
T or F: Ionic compounds form crystals instead of individual molecules?
|
true
|
|
|
give an Example of an ionic compound
|
NaCl (sodium chloride)
|
|
|
Covalent bonds are formed by ?
|
the sharing of two or more electrons
|
|
|
Electron sharing produces?
|
molecules
|
|
|
Electrons shared equally between atoms produce
|
nonpolar molecules
|
|
|
Unequal sharing of electrons produces
|
polar molecules
|
|
|
Atoms with six or seven valence shell electrons are
|
electronegative
|
|
|
Atoms with one or two valence shell electrons are
|
electropositive
|
|
|
T or F: Reactive elements do not have their outermost energy level fully occupied by electrons
|
True
|
|
|
T or F:Inert elements do have their outermost energy level fully occupied by electrons
|
true
|
|
|
name 4 characteristics of hydogen bond
|
1. Too weak to bind atoms together
2. Common in dipoles such as water 3. Responsible for surface tension in water 4. Important as intramolecular bonds, giving the molecule a three-dimensional shape |
|
|
Name 2 facts about Chemical Reactions
|
1. Occur when chemical bonds are formed, rearranged, or broken
2. Are written in symbolic form using chemical equations |

